Abstract
Aims
Chronic inflammation of autoimmune diseases, including type 1 diabetes (T1D), is mainly mediated by memory T(Tm) cells, predominantly effector memory T (Tem) cells. The roles of the programmed death-1 (PD-1) receptor on lymphocytes have been well studied in tumor and other infection models. However, little is known about the relationship between the expression of PD-1 on CD8+ Tem cells and the pathogenesis of T1D.
Methods
A total of 52 patients diagnosed with T1D and 39 gender-, age-, and ethnically matched health control individuals were enrolled in this study. Peripheral blood mononuclear cells from these individuals were isolated and analyzed by flow cytometry. We evaluated the frequencies of PD-1+ CD8+ memory T cell subsets from patients' peripheral blood with T1D and the spleen cells of nonobese diabetic (NOD) mice in the present study. We also investigated the effects of blocking PD-1/PD-L1 pathway on islet’s inflammation in NOD mice.
Results
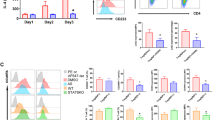
Frequencies of PD-1+ CD8+ Tem cells were decreased significantly in PBMC of patients with T1D (40.73 ± 12.72 vs 47.43 ± 15.56, *p < 0.05). The frequencies of PD-1+ CD8+ Tem cells were decreased in patients with T1D who were positive for two or more autoantibodies compared with the patients with one autoantibody (13.46% vs 46.95 ± 12.72%, *p < 0.05). Meanwhile, the frequencies of PD-1+ CD8+ central memory T (Tcm) cells were also significantly decreased in patients with two or more autoantibodies compared with other groups (≥ 2AAb vs HC 33.1 ± 8.92% vs 43.71 ± 11.78%, *p < 0.05; ≥ 2AAb vs AAb—33.1 ± 8.92% vs 41.65 ± 11.2%, *p < 0.05; ≥ 2AAb vs 1AAb 33.1 ± 8.92% vs 48.09 ± 10.58%, ***p < 0.001). The frequencies of PD-1+CD8+ Tem cells were positively correlated with fasting serum C-peptide levels (r = 0.4308, *p < 0.05) and C-peptide levels 2 h after meal in T1D patients (r = 0.5723, **p < 0.01). The frequencies of PD-1+CD8+ Tcm cells were only negatively correlated with the levels of HbA1c (r = − 0.2992, *p < 0.05). Similarly, the frequencies of PD-1+CD8+ Tem were significantly decreased in intervention group (anti-mouse PD-1 mAb) compared with the control group (14.22 ± 6.455% vs 27.69 ± 9.837%, *p < 0.05). Pathologically, CD8, PD-1 and PD-L1 were strongly expressed in the islets of diabetic mice after PD-1 blockade.
Conclusions
It is the first report of the expression of PD-1 on CD8+ Tem cells in T1D in the present study. Our observations suggest that the PD-1/PD-L1 signal pathway on CD8+ Tem cells of T1D subjects might identify a new pathway for delaying the occurrence and development by inhibiting autoimmunity.






Similar content being viewed by others
Availability of data and materials
The data are available on request from the authors.
References
DiMeglio LA, Evans-Molina C, Oram RA (2018) Type 1 diabetes. Lancet (London, England) 391(10138):2449–2462
Sarikonda G, Pettus J, Phatak S et al (2014) CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J Autoimmun 50:77–82
Culina S, Lalanne AI, Afonso G et al (2018) Islet-reactive CD8 T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol 3(20):eaao4013
Skowera A, Ladell K, McLaren JE et al (2015) Beta-cell-specific CD8 T cell phenotype in type 1 diabetes reflects chronic autoantigen exposure. Diabetes 64(3):916–925
Kumar BV, Connors TJ, Farber DL (2018) Human T cell development, localization, and function throughout life. Immunity 48(2):202–213
Akondy RS, Fitch M, Edupuganti S et al (2017) Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552(7685):362–367
Youngblood B, Hale JS, Kissick HT et al (2017) Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552(7685):404–409
Gattinoni L, Lugli E, Ji Y et al (2011) A human memory T cell subset with stem cell-like properties. Nat Med 17(10):1290–1297
Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401(6754):708–712
Abdelsamed HA, Moustaki A, Fan Y et al (2017) Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. J Exp Med 214(6):1593–1606
Devarajan P, Chen Z (2013) Autoimmune effector memory T cells: the bad and the good. Immunol Res 57:12–22
Chee J, Ko HJ, Skowera A et al (2014) Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J Immunol 192(2):572–580
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11(11):3887–3895
Boussiotis VA (2016) Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 375(18):1767–1778
Ni L, Dong C (2017) New checkpoints in cancer immunotherapy. Immunol Rev 276(1):52–65
Colli ML, Hill JLE, Marroqui L et al (2018) PD-L1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-alpha and-gamma via IRF1 induction. EBioMedicine 36:367–375
Ansari M, Salama A, Chitnis T et al (2003) The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 198(1):63–69
Clotman K, Janssens K, Specenier P, Weets I, De Block CEM (2018) Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab 103(9):3144–3154
Pauken K, Godec J, Odorizzi P et al (2020) The PD-1 pathway regulates development and function of memory CD8 T cells following respiratory viral infection. Cell Rep 31(13):107827
Fujisawa R, Haseda F, Tsutsumi C et al (2015) Low programmed cell death-1 (PD-1) expression in peripheral CD4+T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol 180(3):452–457
Wong F, Wen L (2020) A predictive CD8 T cell phenotype for T1DM progression. Nat Rev Endocrinol 16(4):198–199
Monti P, Heninger AK, Bonifacio E (2009) Differentiation, expansion, and homeostasis of autoreactive T cells in type 1 diabetes mellitus. Curr Diab Rep 9(2):113–118
Oling V, Reijonen H, Simell O, Knip M, Ilonen J (2012) Autoantigen-specific memory CD4+ T cells are prevalent early in progression to Type 1 diabetes. Cell Immunol 273(2):133–139
McGinty JW, Chow IT, Greenbaum C, Odegard J, Kwok WW, James EA (2014) Recognition of posttranslationally modified GAD65 epitopes in subjects with type 1 diabetes. Diabetes 63(9):3033–3040
Chow IT, Yang J, Gates TJ et al (2014) Assessment of CD4+ T cell responses to glutamic acid decarboxylase 65 using DQ8 tetramers reveals a pathogenic role of GAD65 121–140 and GAD65 250–266 in T1D development. PLoS ONE 9(11):e112882
Roep B, Peakman M (2012) Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med 2(4):a007781
Herold KC (2013) Restoring immune balance in type 1 diabetes. Lancet Diabetes Endocrinol 1(4):261–263
Zou W, Wolchok JD, Chen L (2016) PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Science translational medicine 8(328):328rv4
Littman D (2015) Releasing the brakes on cancer immunotherapy. Cell 162(6):1186–1190
Ribas A (2012) Tumor immunotherapy directed at PD-1. N Engl J Med 366(26):2517–2519
Yeo L, Woodwyk A, Sood S et al (2018) Autoreactive T effector memory differentiation mirrors beta cell function in type 1 diabetes. J Clin Investig 128(8):3460–3474
Orban T, Beam CA, Xu P et al (2014) Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes 63(10):3449–3457
Sharpe A, Pauken K (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18(3):153–167
Mellati M, Eaton KD, Brooks-Worrell BM et al (2015) Anti-PD-1 and Anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care 38(9):e137–e138
Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T (2015) Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA 102(33):11823–11828
Ribas A, Shin D, Zaretsky J et al (2016) PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res 4(3):194–203
Kuric E, Seiron P, Krogvold L et al (2017) Demonstration of tissue resident memory CD8 T cells in insulitic lesions in adult patients with recent-onset type 1 diabetes. Am J Pathol 187(3):581–588
Barber D, Wherry E, Masopust D et al (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439(7077):682–687
Kamphorst A, Pillai R, Yang S et al (2017) Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 114(19):4993–4998
Wang C, Chou F, Chu C et al (2008) Protective role of programmed death 1 ligand 1 (PD-L1) in nonobese diabetic mice: the paradox in transgenic models. Diabetes 57(7):1861–1869
Nojima I, Eikawa S, Tomonobu N et al (2020) Dysfunction of CD8+PD-1+ T cells in type 2 diabetes caused by the impairment of metabolism-immune axis. Sci Rep 10(1):14928
Acknowledgements
The authors thank all patients and health providers involved in this study. The work was supported by grants from the National Natural Science Foundation of China (Grant numbers 82070814), Scientific Research Project of Jiangsu Health Commission (Grant Numbers H2019043), Scientific Research Project of Suzhou Health Commission (Grant Numbers SYS2019070), Open project of the State Key Laboratory of Radiation Medicine and Radiation Protection jointly built by the Ministry and the province (Grant Numbers GZK1202025), Second Affiliated Hospital of Soochow University Science Foundation (Grant numbers SDFEYQN1912) and Suzhou Science and Education Health Project (Grant Numbers kjxw2019015).
Funding
The work was supported by grants from the National Natural Science Foundation of China (Grant numbers 82070814), Scientific Research Project of Jiangsu Health Commission (Grant Numbers H2019043), Scientific Research Project of Suzhou Health Commission (Grant Numbers SYS2019070), Open project of the State Key Laboratory of Radiation Medicine and Radiation Protection jointly built by the Ministry and the province (Grant Numbers GZK1202025), Second Affiliated Hospital of Soochow University Science Foundation (Grant numbers SDFEYQN1912), and Suzhou Science and Education Health Project (Grant Numbers kjxw2019015). These funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Author information
Authors and Affiliations
Contributions
CF and JH conceived and designed the study. YMS and YHK analyzed the data and drafted the article. YZ, JJG, QYS, SCL, HMG, and YTH contributed to acquisition of the data. CPL, LC, SSD, YH, CF, and JH revised the article critically for important intellectual content and interpreted the data. All authors read and approved the final version for submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient.
Additional information
Managed By Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yimei Shan and Yinghong Kong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shan, Y., Kong, Y., Zhou, Y. et al. Decreased expression of programmed death-1 on CD8+ effector memory T lymphocytes correlates with the pathogenesis of type 1 diabetes. Acta Diabetol 58, 1239–1249 (2021). https://doi.org/10.1007/s00592-021-01711-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01711-z