Abstract
Aims
To compare the blood pressure (BP)-lowering efficacy of a chlorthalidone/amiloride combination pill with losartan, during initial management of JNC 7 Stage I hypertension in patients with type 2 diabetes mellitus.
Methods
In an a priori subgroup analysis of a randomized, double-blind, controlled trial, volunteers aged 30–70 years, with stage I hypertension and diabetes mellitus, were randomized to 12.5/2.5 mg of chlorthalidone/amiloride (N = 47) or 50 mg of losartan (N = 50), and followed for 18 months in 21 clinical centers. If BP remained uncontrolled after three months, study medication dose was doubled, and if uncontrolled after six months, amlodipine (5 and 10 mg) and propranolol (40 and 80 mg BID) were added as open label drugs in a progressive fashion.
Results
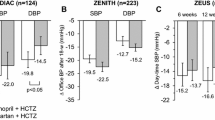
Systolic BP decreased to a greater extent in participants allocated to diuretics compared to losartan (P < 0.001). After 18 months of follow-up, systolic BP was 128.4 ± 10.3 mmHg in the diuretic group versus 133.5 ± 8.0 in the losartan group (P < 0.01). In the diuretic group, 36 out of 43 participants (83.7%) had a JNC 7 normal BP, compared to 31/47 (66%) in the losartan group (P = 0.089). Serum cholesterol was higher in the diuretic arm at the end of the trial. Other biochemical parameters and reports of adverse events did not differ by treatment.
Conclusions
Treatment of hypertension based on a combination of chlorthalidone and amiloride is more effective for BP lowering compared to losartan in patients with diabetes mellitus and hypertension.
Trial registration
Clinical trials registration number: NCT00971165


Similar content being viewed by others
Availability of data and material (data transparency)
All datasets on which the conclusions of the paper rely will be available to in publicly available repository (https://www.lume.ufrgs.br/).
Change history
01 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00592-021-01689-8
References
Williams B, Mancia G, Spieng W et al (2018) ESC scientific document group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 39:3021–3104. https://doi.org/10.1093/eurheartj/ehy339
Thomopoulos C, Parati G, Zanchetti A (2017) Effects of blood-pressure-lowering treatment on outcome incidence in hypertension: 10 - Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J Hypertens 35:922–944. https://doi.org/10.1097/HJH.0000000000001276
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison-Himmelfarb C et al (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension 71:e13–e115. https://doi.org/10.1161/HYP.0000000000000065
Fuchs FD, DiNicolantonio JJ (2015) Angiotensin receptor blockers for prevention of cardiovascular disease: where does the evidence stand? Open Heart 2:e000236. https://doi.org/10.1136/openhrt-2014-000236
Bangalore S, Kumar S, Wetterslev J, Messerli FH (2011) Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ 342:d2234. https://doi.org/10.1136/bmj.d2234
van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ et al (2012) Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158 998 patients. Eur Heart J 33:2088–2097. https://doi.org/10.1093/eurheartj/ehs075
Cheng J, Zhang W, Zhang X, Han F, Li X, He X et al (2014) Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med 174:773–785. https://doi.org/10.1001/jamainternmed.2014.348
Fuchs FD, Scala LC, Vilela-Martin JF, Bandeira-de-Mello R, Mosele F, Whelton PK et al (2016) Effectiveness of chlorthalidone/amiloride versus losartan in patients with stage I hypertension: results from the PREVER-Treatment randomized trial. J Hypertens 34:798–806. https://doi.org/10.1097/HJH.0000000000000837
Fuchs FD, Fuchs SC, Moreira LB, Gus M, Nóbrega AC, Poli-de-Figueiredo CE et al (2011) A comparison between diuretics and angiotensin-receptor blocker agents in patients with stage I hypertension (PREVER-Treatment trial): study protocol for a randomized double-blind controlled trial. Trials 12:53. https://doi.org/10.1186/1745-6215-12-53
Law MR, Morris JK, Wald NJ (2009) Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338:b1665. https://doi.org/10.1136/bmj.b1665
Bottino LG, Fuchs FD (2020) The role of angiotensin receptor blockers in CVD risk management. Expert Rev Cardiovasc Ther 8:181–185. https://doi.org/10.1080/14779072.2020.1750369
Fuchs FD, Whelton PK (2020) High Blood Pressure and Cardiovascular Disease. Hypertension 75:285–292. https://doi.org/10.1161/HYPERTENSIONAHA.119.14240
Fuchs FD (ed) (2018) Essentials of Hypertension. Springer, Cham
Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P (2001) The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345:870–878. https://doi.org/10.1056/NEJMoa011489
Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE, Parving HH et al (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Eng J Med 345(12):861–869. https://doi.org/10.1056/NEJMoa011161
Funding
This study was funded by grants from the Department of Science and Technology (DECIT), Health Ministry; National Council of Research (CNPq) and Agency for Funding of Studies and Projects (FINEP; Number 01080606/01), Science and Technology Ministry; Funding of Incentive to Research of Hospital de Clinicas de Porto Alegre (FIPE-HCPA: GPPG 08-621), all in Brazil. The sponsors had no participation in the design and conduct of the study, preparation and approval of the manuscript.
Author information
Authors and Affiliations
Contributions
SCF and FDF have full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All of the authors made substantial contributions to the conception of the study, and/or the acquisition, analysis, or interpretation of study data, and/or drafting or revision of the manuscript, and all authors approved the final version of the manuscript and agreed to be accountable for all aspects of the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of the Hospital de Clinicas de Porto Alegre (GPPG 08-621), which is accredited by the Office of Human Research Protections as an Institutional Review Board, and by the ethics committee at each medical center. The PREVER-Treatment trial was conducted in 21 academic medical centers in Brazil, and was approved by the Research Ethics Board of each participant institution.
Informed consent
Eligible participants were enrolled in the trial and gave written informed consent prior to their inclusion in the study.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hilton Chaves, Abrahão Afiune Neto: in memorial.
Rights and permissions
About this article
Cite this article
Fuchs, F.D., Scala, L.C.N., Vilela-Martin, J.F. et al. Effectiveness of chlorthalidone/amiloride versus losartan in patients with stage I hypertension and diabetes mellitus: results from the PREVER-treatment randomized controlled trial. Acta Diabetol 58, 215–220 (2021). https://doi.org/10.1007/s00592-020-01611-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01611-8