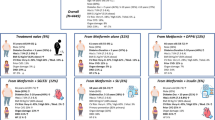
Abstract
Aims
Our study aimed to analyse treatment strategies after failure of initial metformin mono-therapy in adult patients with type-2 diabetes (T2DM).
Methods
The DIVE and DPV databases were combined and 16,681 adult patients with T2DM and metformin mono-therapy identified. Patients were analysed depending on whether metformin was continued (MET), or whether it was combined with other oral antidiabetics (OAD), with GLP-1 antagonists (GLP-1) or with basal insulin (BOT/BOT plus). Metabolic control, body weight and hypoglycaemia, micro- and macro-vascular events were compared within 1 year.
Results
A total of 11,911 (71%) participants continued MET until the end of the observation period, 3334 (20.0%) were intensified using OAD, 579 (3%) started on GLP-1, and 857 (5%) were initiated on BOT/BOTplus. Predictors of OAD and BOT/BOTplus therapy were elevated HbA1c, longer diabetes duration and the presence of micro- and macro-vascular diseases, while GLP-1 therapy was predicted by younger age, female sex, higher body weight and shorter diabetes duration. Micro- and macro-vascular diseases were negative predictors of GLP-1 therapy. Effects on HbA1c were highest in the BOT/BOTplus and OAD group, while GLP-1 treatment had the best effect on body weight.
Conclusions
BOT/BOTplus and OAD show good HbA1c reduction even in patients with longer diabetes duration and in older patients. GLP-1 therapy is effective concerning weight loss in overweight patients and is more often used in females and patients with shorter diabetes duration. Interestingly, despite several studies showing positive effects on micro- and macro-vascular outcomes, prevalent macro-vascular diseases are no predictors of GLP-1 use.

Similar content being viewed by others
References
Davies MJ, D’Alessio DA, Fradkin J et al (2018) Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41:2669–2701. https://doi.org/10.2337/dci18-0033
American Diabetes A (2019) 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care 42:S90–S102. https://doi.org/10.2337/dc19-S009
Vijan S, Sussman JB, Yudkin JS, Hayward RA (2014) Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med 174:1227–1234. https://doi.org/10.1001/jamainternmed.2014.2894
Danne T, Kaltheuner M, Koch A et al (2013) “DIabetes versorgungs-evaluation” (DIVE)—a national quality assurance initiative at physicians providing care for patients with diabetes. Dtsch Med Wochenschr 138:934–939. https://doi.org/10.1055/s-0033-1343144
Grabert M, Schweiggert F, Holl RW (2002) A framework for diabetes documentation and quality management in Germany: 10 years of experience with DPV. Comput Methods Programs Biomed 69:115–121
DCCT Research Group (1987) Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. The DCCT Research Group. Clin Chem 33:2267–2271
Rosenbauer J, Dost A, Karges B et al (2012) Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care 35:80–86. https://doi.org/10.2337/dc11-0993
Bohn B, Kerner W, Seufert J et al (2016) Trend of antihyperglycaemic therapy and glycaemic control in 184,864 adults with type 1 or 2 diabetes between 2002 and 2014: analysis of real-life data from the DPV registry from Germany and Austria. Diabetes Res Clin Pract 115:31–38. https://doi.org/10.1016/j.diabres.2016.03.008
Workgroup on Hypoglycemia ADA (2005) Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 28:1245–1249. https://doi.org/10.2337/diacare.28.5.1245
Bethel MA, Patel RA, Merrill P et al (2018) Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 6:105–113. https://doi.org/10.1016/S2213-8587(17)30412-6
Desai NR, Shrank WH, Fischer MA et al (2012) Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med 125(302):e301–e307. https://doi.org/10.1016/j.amjmed.2011.07.033
Azoulay L, Suissa S (2017) Sulfonylureas and the risks of cardiovascular events and death: a methodological meta-regression analysis of the observational studies. Diabetes Care 40:706–714. https://doi.org/10.2337/dc16-1943
Hirst JA, Farmer AJ, Dyar A, Lung TW, Stevens RJ (2013) Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta-analysis. Diabetologia 56:973–984. https://doi.org/10.1007/s00125-013-2856-6
Zhang Y, McCoy RG, Mason JE, Smith SA, Shah ND, Denton BT (2014) Second-line agents for glycemic control for type 2 diabetes: are newer agents better? Diabetes Care 37:1338–1345. https://doi.org/10.2337/dc13-1901
(2013) Canaglifozin (Invokana) for type 2 diabetes. Med Lett Drugs Ther 55:37–39
Hartmann B, Bramlage P, Schneider S, Tschope D, Gitt AK (2015) Impact of body weight on antidiabetic treatment and predictors of weight control under real-world conditions: a 2-year follow-up of DiaRegis cohort. Acta Diabetol 52:1093–1101. https://doi.org/10.1007/s00592-015-0794-0
Acknowledgements
We thank all participating centres of the DPV and DIVE initiatives, especially those who collaborated in this investigation.
Funding
The DPV registry was supported by the European Foundation for the Study of Diabetes (EFSD). Further financial support was provided by the German Diabetes Society (DDG) and the German Centre for Diabetes Research (DZD). The DIVE registry received funding from Sanofi, AstraZeneca, Bayer and Abbott. Funders were not involved in study design, the collection, analysis and interpretation of data, the writing of the report and the decision to submit the article for publication.
Author information
Authors and Affiliations
Consortia
Contributions
FJW, MD, SS and MP collected data and contributed to discussion and finalization of the manuscript. BH, GvM and PB designed the analysis, drafted the manuscript and created figures. SL and RWH did the statistical analyses. SL, FJW, MD, MP, SS, JS and RWH contributed to the discussion and reviewed/edited the manuscript. SL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final manuscript submitted.
Corresponding author
Ethics declarations
Conflict of interest
JS reports grants and personal fees from Abbott, AstraZeneca and Sanofi, outside the submitted work. PB reports to have received consultancy honoraria from Sanofi and Abbott. BH, SL, GvM, FJW, MD, MP and SS have no competing interests to disclose.
Ethical standard
The registry was conducted in accordance with Good Epidemiology Practice and applicable regulatory requirements. The protocol of DIVE registry was approved by the ethics committee of the Medical School of Hannover (approval no. 6003), the protocol of DPV was approved by the ethics committee of the University of Ulm (approval no. 202/09), and data collection was approved by local review board.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patients being enrolled into this registry provided written informed consent.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hartmann, B., Lanzinger, S., van Mark, G. et al. Treatment intensification strategies after initial metformin therapy in adult patients with type-2 diabetes: results of the DPV and DIVE registries. Acta Diabetol 57, 229–236 (2020). https://doi.org/10.1007/s00592-019-01409-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01409-3