Abstract
Aims
Oxidative stress has been considered to contribute to the development of obesity-related metabolic disorders including insulin resistance. To the contrary, deficiency of an anti-oxidizing enzyme, glutathione peroxidase (GPx)-1, was reported to enhance insulin signaling, suggesting that oxidative stress may inhibit the development of type 2 diabetes. However, the beneficial effects of the absence of GPx-1 in metabolic homeostasis, including body weight control, have not yet been clearly manifested. To clarify the relationship between oxidative stress and obesity-related metabolic disorders, we investigated another mouse deficient with both GPx-1 and catalase (Cat).
Methods
C57BL/6J wild-type and GPx-1−/− × Cat−/− mice were fed with a high-fat diet (60% fat) or a normal chow diet for 16 weeks and were investigated for metabolic and histological studies.
Results
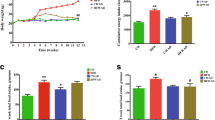
Body weight gain was significantly reduced, and glucose metabolism as well as hepatic steatosis was obviously improved in the GPx-1−/− × Cat−/− mice. The serum levels of insulin and total cholesterol were also significantly lowered. For the underlying mechanism, inflammation was attenuated and expression of markers for fat browning was enhanced in the visceral white adipose tissues.
Conclusions
Oxidative stress due to deficiency of GPx-1 and Cat may improve obesity-related metabolic disorders through attenuation of inflammation and fat browning.






Similar content being viewed by others
References
WHO (2018) Obesity and overweight 2018. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 16 Feb 2018
Al-Goblan AS, Al-Alfi MA, Khan MZ (2014) Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 7:587–591
Milic S, Lulic D, Stimac D (2014) Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol 20:9330–9337
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M et al (2018) Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15:11–20
Oseini AM, Sanyal AJ (2017) Therapies in non-alcoholic steatohepatitis (NASH). Liver Int 37(Suppl 1):97–103
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y et al (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761
Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D et al (2003) Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23:434–439
DeMarco VG, Johnson MS, Whaley-Connell AT, Sowers JR (2010) Cytokine abnormalities in the etiology of the cardiometabolic syndrome. Curr Hypertens Rep 12:93–98
Keane KN, Cruzat VF, Carlessi R, de Bittencourt PI Jr, Newsholme P P (2015) Molecular events linking oxidative stress and inflammation to insulin resistance and beta-cell dysfunction. Oxid Med Cell Longev 2015:181643
Youn JY, Siu KL, Lob HE, Itani H, Harrison DG, Cai H (2014) Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 63:2344–2355
Veal EA, Day AM, Morgan BA (2007) Hydrogen peroxide sensing and signaling. Mol Cell 26:1–14
Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S et al (2009) Reactive oxygen species enhance insulin sensitivity. Cell Metab 10:260–272
Merry TL, Tran M, Dodd GT, Mangiafico SP, Wiede F, Kaur S et al (2016) Hepatocyte glutathione peroxidase-1 deficiency improves hepatic glucose metabolism and decreases steatohepatitis in mice. Diabetologia 59:2632–2644
Haas JT, Staels B (2016) An oxidative stress paradox: time for a conceptual change? Diabetologia 59:2514–2517
Merry TL, Tran M, Stathopoulos M, Wiede F, Fam BC, Dodd GT et al (2014) High-fat-fed obese glutathione peroxidase 1-deficient mice exhibit defective insulin secretion but protection from hepatic steatosis and liver damage. Antioxid Redox Signal 20:2114–2129
Asghar A, Sheikh N (2017) Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol 315:18–26
Cipolletta D (2014) Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology 142:517–525
Wagner NM, Brandhorst G, Czepluch F, Lankeit M, Eberle C, Herzberg S et al (2013) Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity (Silver Spring) 21:461–468
Mito N, Hosoda T, Kato C, Sato K (2000) Change of cytokine balance in diet-induced obese mice. Metabolism 49:1295–1300
Vargas R, Ryder E, Diez-Ewald M, Mosquera J, Duran A, Valero N et al (2016) Increased C-reactive protein and decreased Interleukin-2 content in serum from obese individuals with or without insulin resistance: associations with leukocyte count and insulin and adiponectin content. Diabetes Metab Syndr 10:S34–S41
Won HY, Sohn JH, Min HJ, Lee K, Woo HA, Ho YS et al (2010) Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid Redox Signal 13:575–587
de Haan JB, Witting PK, Stefanovic N, Pete J, Daskalakis M, Kola I et al (2006) Lack of the antioxidant glutathione peroxidase-1 does not increase atherosclerosis in C57BL/J6 mice fed a high-fat diet. J Lipid Res 47:1157–1167
Kim HR, Lee A, Choi EJ, Hong MP, Kie JH, Lim W et al (2014) Reactive oxygen species prevent imiquimod-induced psoriatic dermatitis through enhancing regulatory T cell function. PLoS ONE 9:e91146
Kim HR, Lee A, Choi EJ, Kie JH, Lim W, Lee HK et al (2014) Attenuation of experimental colitis in glutathione peroxidase 1 and catalase double knockout mice through enhancing regulatory T cell function. PLoS ONE 9:e95332
Kim SJ, Lee JW, Jung YS, Kwon DY, Park HK, Ryu CS et al (2009) Ethanol-induced liver injury and changes in sulfur amino acid metabolomics in glutathione peroxidase and catalase double knockout mice. J Hepatol 50:1184–1191
Takahashi Y, Fukusato T (2014) Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 20:15539–15548
Won HY, Jang EJ, Lee K, Oh S, Kim HK, Woo HA et al (2013) Ablation of peroxiredoxin II attenuates experimental colitis by increasing FoxO1-induced Foxp3+ regulatory T cells. J Immunol 191:4029–4037
Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, Holmdahl R (2004) Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci USA 101:12646–12651
Won HY, Jang EJ, Min HJ, Hwang ES (2011) Enhancement of allergen-induced airway inflammation by NOX2 deficiency. Immune Netw 11:169–174
De Ravin SS, Naumann N, Cowen EW, Friend J, Hilligoss D, Marquesen M et al (2008) Chronic granulomatous disease as a risk factor for autoimmune disease. J Allergy Clin Immunol 122:1097–1103
Costford SR, Castro-Alves J, Chan KL, Bailey LJ, Woo M, Belsham DD et al (2014) Mice lacking NOX2 are hyperphagic and store fat preferentially in the liver. Am J Physiol Endocrinol Metab 306:E1341–E1353
Heit C, Marshall S, Singh S, Yu X, Charkoftaki G, Zhao H et al (2017) Catalase deletion promotes prediabetic phenotype in mice. Free Radic Biol Med 103:48–56
Shin SK, Cho HW, Song SE, Bae JH, Im SS, Hwang I et al (2019) Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflugers Arch 471:829–843
Kim SH, Plutzky J (2016) Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders. Diabetes Metab J 40:12–21
Funding
This study was supported by a grant from Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0050).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical standard statement
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Institutional Animal Care and Use Committee of Ewha Womans University Graduate School of Medicine, Permit Number: 10-0133).
Informed consent
As this study includes only animal study, informed consent is not required.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, HR., Choi, EJ., Kie, JH. et al. Deficiency of glutathione peroxidase-1 and catalase attenuated diet-induced obesity and associated metabolic disorders. Acta Diabetol 57, 151–161 (2020). https://doi.org/10.1007/s00592-019-01388-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01388-5