Abstract
Aims
To investigate the occurrence of myopathy with oral glucose-lowering drugs (OGLDs) and statins, with a focus on dipeptidyl peptidase-4 inhibitors (DPP4-is).
Methods
The FDA adverse event reporting system (FAERS) was queried (2004–2016) to compare the proportion of adverse events with OGLDs, alone and in combination with statins, using the reporting odds ratio (ROR) with relevant 95% confidence interval (95%Cl), adjusted for sex, age and concomitant presence of other OGLDs/lipid-lowering drugs. Drug–drug interaction is claimed whenever the frequency of an event is enhanced by combination treatment. Consistency/robustness of findings was tested by applying additive/multiplicative models and accounting for competition bias (i.e., adverse events previously known to be associated with OGLDs were removed).
Results
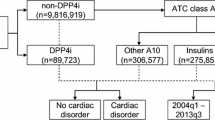
Over a 13-year period, we retrieved 142,888 cases of myopathy. The use of DPP4-is alone was not associated with higher reporting of myopathy (no. of cases = 4898; adjusted ROR = 1.00; 95%CI = 0.96–1.04), with the notable exclusion of vildagliptin (262; 1.64; 1.42–1.88). No increased occurrence emerged when used in combination with statins, with consistent findings from additive/multiplicative models for all DPP4-is. Likewise, no increased reporting was found for other OGLDs (28,964; 0.64; 0.62–0.67); data on the interaction with statins were consistent/robust across analyses only for sulfonylureas (the interaction is likely and biologically plausible) and sodium glucose cotransporter-2 inhibitors.
Conclusions
Real-world FAERS data do not raise concern for muscular toxicity with DPP4-is in combination with statins, making a drug interaction very unlikely.
Similar content being viewed by others
References
Bhome R, Penn H (2012) Rhabdomyolysis precipitated by a sitagliptin-atorvastatin drug interaction. Diabet Med 29(5):693–694
Khan MW, Kurian S, Bishnoi R (2016) Acute-onset rhabdomyolysis secondary to sitagliptin and atorvastatin interaction. Int J Gen Med 9:103–106
Kao DP, Kohrt HE, Kugler J (2008) Renal failure and rhabdomyolysis associated with sitagliptin and simvastatin use. Diabet Med 25(10):1229–1230
DiGregorio RV, Pasikhova Y (2009) Rhabdomyolysis caused by a potential sitagliptin-lovastatin interaction. Pharmacotherapy 29(3):352–356
European Medicines Agency (2014) Vildagliptin; Vildagliptin, metformin—Rhabdomyolysis. In PRAC recommendations on signals. Adopted at the PRAC Meeting of 10–13 June 2014. London, U.K., European Medicines Agency, pp 8–9 [internet]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/PRAC_recommendation_on_signal/2014/07/WC500169486.pdf
Strandell J, Caster O, Bate A, Noren N, Edwards IR (2011) Reporting patterns indicative of adverse drug interactions: a systematic evaluation in VigiBase. Drug Saf 34(3):253–266
Fadini GP, Bonora BM, Mayur S, Rigato M, Avogaro A (2018) Dipeptidyl peptidase-4 inhibitors moderate the risk of genitourinary tract infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab 20(3):740–744
Fadini GP, Sarangdhar M, Avogaro A (2018) Pharmacovigilance evaluation of the association between DPP-4 inhibitors and heart failure: stimulated reporting and moderation by drug interactions. Diabet Ther 9(2):851–861
Labat V, Arnaud M, Miremont-Salame G, Salvo F, Begaud B, Pariente A (2017) Risk of myopathy associated with DPP-4 inhibitors in combination with statins: a disproportionality analysis using data from the WHO and French spontaneous reporting databases. Diabet Care 40(3):e27–e29
Tarapues M, Cereza G, Figueras A (2013) Association of musculoskeletal complaints and gliptin use: review of spontaneous reports. Pharmacoepidemiol Drug Saf 22(10):1115–1118
Raschi E, Moretti U, Salvo F et al (2018) Evolving roles of spontaneous reporting systems to assess and monitor drug safety. In: Kothari CS, Shah M, Patel RM (eds) Pharmacovigilance. IntechOpen. https://doi.org/10.5772/intechopen.79986
Raschi E, Poluzzi E, Salvo F, et al. (2018) Pharmacovigilance of sodium-glucose co-transporter-2 inhibitors: what a clinician should know on disproportionality analysis of spontaneous reporting systems. Nutr Metab Cardiovasc Dis 28(6):533–542
Poluzzi E, Raschi E, Piccinni C, De Ponti F (2012) Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA adverse event reporting system (AERS). In: Karahoca A (ed) Data mining applications in engineering and medicine. InTech, Croatia, pp 265–302
Fukazawa C, Hinomura Y, Kaneko M, Narukawa M (2018) Significance of data mining in routine signal detection: analysis based on the safety signals identified by the FDA. Pharmacoepidemiol Drug Saf 27(12):1402–1408
Harpaz R, DuMouchel W, LePendu P, et al. (2013) Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin Pharmacol Ther 93(6):539–546
Bate A, Evans SJ (2009) Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 18(6):427–436
Sakaeda T, Tamon A, Kadoyama K, Okuno Y (2013) Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci 10(7):796–803
Geniaux H, Assaf D, Miremont-Salame G, Raspaud B, Gouverneur A, Robinson P et al (2014) Performance of the standardised MedDRA(R) queries for case retrieval in the French spontaneous reporting database. Drug Saf 37(7):537–542
van Puijenbroek EP, Egberts AC, Heerdink ER, Leufkens HG (2000) Detecting drug-drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol 56(9–10):733–738
Van Puijenbroek EP, Egberts AC, Meyboom RH, Leufkens HG (1999) Signalling possible drug-drug interactions in a spontaneous reporting system: delay of withdrawal bleeding during concomitant use of oral contraceptives and itraconazole. Br J Clin Pharmacol 47(6):689–693
Yue Z, Shi J, Jiang P, Sun H (2014) Acute kidney injury during concomitant use of valacyclovir and loxoprofen: detecting drug-drug interactions in a spontaneous reporting system. Pharmacoepidemiol Drug Saf 23(11):1154–1159
Capogrosso Sansone A, Convertino I, Galiulo MT, et al. (2017) Muscular adverse drug reactions associated with proton pump inhibitors: a disproportionality analysis using the Italian national network of pharmacovigilance database. Drug Saf 40(10):895–909
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC (2002) A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 11(1):3–10
Thakrar BT, Grundschober SB, Doessegger L (2007) Detecting signals of drug-drug interactions in a spontaneous reports database. Br J Clin Pharmacol 64(4):489–495
Raschi E, Piccinni C, Poluzzi E, Marchesini G, De Ponti F (2013) The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol 50(4):569–577
Fadini GP, Bonora BM, Avogaro A (2017) SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA adverse event reporting system. Diabetologia 60(8):1385–1389
Motola D, Piccinni C, Biagi C, et al. (2012) Cardiovascular, ocular and bone adverse reactions associated with thiazolidinediones: a disproportionality analysis of the US FDA adverse event reporting system database. Drug Saf 35(4):315–323
Piccinni C, Motola D, Marchesini G, Poluzzi E (2011) Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabet Care 34(6):1369–1371
Salvo F, Leborgne F, Thiessard F, Moore N, Begaud B, Pariente A (2013) A potential event-competition bias in safety signal detection: results from a spontaneous reporting research database in France. Drug Saf 36(7):565–572
Kalaitzoglou E, Fowlkes JL, Popescu I, Thrailkill KM (2018) Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabet/Metab Res Rev 35:e3100
Scheen AJ (2018) The safety of gliptins: updated data in 2018. Expert Opin Drug Saf 17(4):387–405
Sesti G, Avogaro A, Belcastro S et al (2019) Ten years of experience with DPP-4 inhibitors for the treatment of type 2 diabetes mellitus. Acta Diabetol 56(6):605–617
Mascolo A, Rafaniello C, Sportiello L, et al. (2016) Dipeptidyl Peptidase (DPP)-4 inhibitor-induced arthritis/arthralgia: a review of clinical cases. Drug Saf 39(5):401–407
Bouchi R, Fukuda T, Takeuchi T, et al. (2018) Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabet/Metab Res Rev 34(2):e2957
Rizzo MR, Barbieri M, Fava I, et al. (2016) Sarcopenia in elderly diabetic patients: role of dipeptidyl peptidase 4 inhibitors. J Am Med Dir Assoc 17(10):896–901
Bergman AJ, Cote J, Maes A, et al. (2009) Effect of sitagliptin on the pharmacokinetics of simvastatin. J Clin Pharmacol 49(4):483–488
Tornio A, Niemi M, Neuvonen PJ, Backman JT (2012) Drug interactions with oral antidiabetic agents: pharmacokinetic mechanisms and clinical implications. Trends Pharmacol Sci 33(6):312–322
He YL (2012) Clinical pharmacokinetics and pharmacodynamics of vildagliptin. Clin Pharmacokinet 51(3):147–162
Hoffmann P, Martin L, Keselica M, et al. (2017) Acute toxicity of Vildagliptin. Toxicol Pathol 45(1):76–83
Schelleman H, Han X, Brensinger CM, et al. (2014) Pharmacoepidemiologic and in vitro evaluation of potential drug-drug interactions of sulfonylureas with fibrates and statins. Br J Clin Pharmacol 78(3):639–648
Leonard CE, Bilker WB, Brensinger CM, et al. (2016) Severe hypoglycemia in users of sulfonylurea antidiabetic agents and antihyperlipidemics. Clin Pharmacol Ther 99(5):538–547
Acknowledgements
Authors at the University of Bologna are supported by Institutional funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ICA, EP, EF, FS, AP, FDP and ER have no conflicts of interest relevant to the content of the present work. GM reports personal fees and other from Sanofi, personal fees and other from NOVO Nordisk, personal fees and other from Eli-Lilly, other from Astra Zeneca, other from Glaxo, personal fees and other from Janssen, outside the submitted work.
Ethical approval
For this type of study, ethical approval is not required.
Informed Consent
For this type of study, formal consent is not required.
Additional information
Managed by Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Antonazzo, I.C., Poluzzi, E., Forcesi, E. et al. Myopathy with DPP-4 inhibitors and statins in the real world: investigating the likelihood of drug–drug interactions through the FDA adverse event reporting system. Acta Diabetol 57, 71–80 (2020). https://doi.org/10.1007/s00592-019-01378-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01378-7