Abstract
Aims
Poor myometrial contractility has been demonstrated in women at term with diabetes and decreased muscular mitochondrial content and/or function has been extensively implicated in the progression of type 2 diabetes. Alterations of the uterine mitochondrial phenotype in pregnant women with diabetes have yet to be investigated as a causal link to decreased myometrial contractility.
Methods
Observational study of 18 women with diabetes (type 2 and gestational) scheduled for an elective Caesarean section at term with matching controls. A uterine biopsy and fasting blood samples were taken on the day of delivery.
Results
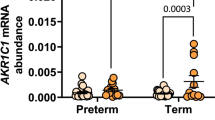
Respiration rates in isolated mitochondria and myometrial mRNA levels of genes related to mitochondrial biogenesis were unaffected by diabetes. Mitochondrial quantity examined by quantification of the complexes of the respiratory chain and histology did not indicate alterations in mitochondrial quantity. Citrate syntase activity was higher (0.31 ± 0.02 vs. 0.24 ± 0.02 U/mg protein, P = 0.008), whereas protein content was lower in women with diabetes compared with the control group (94.6 ± 6.9 vs. 118.6 ± 7.4 mg/g wet wt, P = 0.027). Histological examinations did not support any structural alterations in the myometrium or its mitochondria.
Conclusion
No indication of decreased mitochondrial function, content, morphology, or localization in the myometrium at term in women with diabetes compared with controls was observed. The increase in citrate syntase activity in the myometrium could be explained by the lower protein content in the myometrium, which we suggest is due to alterations in tissue or cellular composition.


Similar content being viewed by others
References
Al-Qahtani S, Heath A, Quenby S et al. (2012) Diabetes is associated with impairment of uterine contractility and high Caesarean section rate. Diabetologia 55(2):489–498. https://doi.org/10.1007/s00125-011-2371-6
Dunne F, Brydon P, Smith K, Gee H (2003) Pregnancy in women with Type 2 diabetes: 12 years outcome data 1990–2002. Diabet Med 20(9):734–738. https://doi.org/10.1046/j.1464-5491.2003.01017
Ehrenberg HM, Durnwald CP, Catalano P, Mercer BM (2004) The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol 191(3):969–974. https://doi.org/10.1016/j.ajog.2004.06.057
Evers IM, de Valk HW, Visser GH (2004) Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ 328(7445):915. https://doi.org/10.1136/bmj.38043.583160.EE
Jensen DM, Damm P, Sorensen B et al. (2003) Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. Am J Obstet Gynecol 189(1):239–244. https://doi.org/10.1067/mob.2003.441
Ovesen PG, Jensen DM, Damm P, Rasmussen S, Kesmodel US (2015) Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. a nation-wide study. J Matern Fetal Neonatal Med 28(14):1720–1724. https://doi.org/10.3109/14767058.2014.966677
Walsh J, Foley M, O’Herlihy C (2011) Dystocia correlates with body mass index in both spontaneous and induced nulliparous labors. J Matern Fetal Neonatal Med 24(6):817–821. https://doi.org/10.3109/14767058.2010.531313
Brown J, Alwan NA, West J et al. (2017) Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev 5:CD011970. https://doi.org/10.1002/14651858.CD011970.pub2
Jensen DM, Damm P, Moelsted-Pedersen L et al. (2004) Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care 27(12):2819–2823. https://doi.org/10.2337/diacare.27.12.2819
McMurtrie EM, Ginsberg GG, Frederick GT, Kirkland JL, Stancel GM, Gardner RM (1985) Effect of a diabetic state on myometrial ultrastructure and isolated uterine contractions in the rat. Proc Soc Exp Biol Med 180(3):497–504
Jawerbaum A, Catafau JR, Gonzalez ET et al. (1996) Eicosanoid production, metabolism and contractile activity in the isolated uterus from non-insulin-dependent diabetic rats during late pregnancy. Prostaglandins 51(5):307–320
Jawerbaum A, Rosello Catafau J, Gonzalez ET et al. (1994) Glucose metabolism, triglyceride and glycogen levels, as well as eicosanoid production in isolated uterine strips and in embryos in a rat model of non-insulin-dependent diabetes mellitus during pregnancy. Prostaglandins 47(2):81–96
Jawerbaum A, Gonzalez ET, Catafau JR et al. (1993) Glucose, glycogen and triglyceride metabolism, as well as prostaglandin production in uterine strips and in embryos from diabetic pregnant rats. Influences of the presence of substrate in the incubation medium. Prostaglandins 46(5):417–431
Di Meo S, Iossa S, Venditti P (2017) Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol 233(1):R15–R42. https://doi.org/10.1530/JOE-16-0598
Goodpaster BH (2013) Mitochondrial deficiency is associated with insulin resistance. Diabetes 62(4):1032–1035. https://doi.org/10.2337/db12-1612
Holloszy JO (2013) “Deficiency” of mitochondria in muscle does not cause insulin resistance. Diabetes 62(4):1036–1040. https://doi.org/10.2337/db12-1107
Ritov VB, Menshikova EV, Azuma K et al. (2010) Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298(1):E49–58. https://doi.org/10.1152/ajpendo.00317.2009
Schrauwen-Hinderling VB, Kooi ME, Hesselink MK et al. (2007) Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 50(1):113–120. https://doi.org/10.1007/s00125-006-0475-1
Holloway GP, Thrush AB, Heigenhauser GJ et al. (2007) Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am J Physiol Endocrinol Metab 292(6):E1782–1789. https://doi.org/10.1152/ajpendo.00639.2006
Heilbronn LK, Gan SK, Turner N, Campbell LV, Chisholm DJ (2007) Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab 92(4):1467–1473. https://doi.org/10.1210/jc.2006-2210
Mootha VK, Lindgren CM, Eriksson KF et al. (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34(3):267–273. https://doi.org/10.1038/ng1180
Patti ME, Butte AJ, Crunkhorn S et al. (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100(14):8466–8471. https://doi.org/10.1073/pnas.1032913100
Hernandez-Alvarez MI, Thabit H, Burns N et al. (2010) Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 33(3):645–651. https://doi.org/10.2337/dc09-1305
Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P (2007) Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 31(8):1302–1310. https://doi.org/10.1038/sj.ijo.0803567
Hastie R, Lappas M (2014) The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta 35(9):673–683. https://doi.org/10.1016/j.placenta.2014.06.368
Qiu C, Hevner K, Abetew D et al. (2013) Mitochondrial DNA copy number and oxidative DNA damage in placental tissues from gestational diabetes and control pregnancies: a pilot study. Clin Lab 59(5–6):655–660
Gam C, Larsen LH, Mortensen OH et al. (2017) Unchanged mitochondrial phenotype, but accumulation of lipids in the myometrium in obese pregnant women. J Physiol 595(23):7109–7122. https://doi.org/10.1113/JP274838
Lauenborg J, Grarup N, Damm P et al. (2009) Common type 2 diabetes risk gene variants associate with gestational diabetes. J Clin Endocrinol Metab 94(1):145–150. https://doi.org/10.1210/jc.2008-1336
Wikstrom M, Ahonen P, Luukkainen T (1975) The role of mitochondria in uterine contractions. FEBS Lett 56(1):120–123
Fritzen AJ, Grunnet N, Quistorff B (2007) Flux control analysis of mitochondrial oxidative phosphorylation in rat skeletal muscle: pyruvate and palmitoyl-carnitine as substrates give different control patterns. Eur J Appl Physiol 101(6):679–689 (Epub 2007 Aug 2024)
Grimpo K, Kutschke M, Kastl A, Meyer CW, Heldmaier G, Exner C, Jastroch M (2014) Metabolic depression during warm torpor in the Golden spiny mouse (Acomys russatus) does not affect mitochondrial respiration and hydrogen peroxide release. Comp Biochem Physiol A Mol Integr Physiol 167:7–14. https://doi.org/10.1016/j.cbpa.2013.09.002
Jarmuszkiewicz W, Woyda-Ploszczyca A, Koziel A, Majerczak J, Zoladz JA (2015) Temperature controls oxidative phosphorylation and reactive oxygen species production through uncoupling in rat skeletal muscle mitochondria. Free Radic Biol Med 83:12–20. https://doi.org/10.1016/j.freeradbiomed.2015.02.012
Perry CG, Kane DA, Lanza IR, Neufer PD (2013) Methods for assessing mitochondrial function in diabetes. Diabetes 62(4):1041–1053. https://doi.org/10.2337/db12-1219
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Kates M (1986) Techniques in Lipidology. Elsevier, New York
Wieland O (1984) Methods of enzymatic analysis, vol VI. Verlag Chemie, Weinheim, pp 504–510
de Melo JF, Aloulou N, Duval JL et al. (2011) Effect of a neonatal low-protein diet on the morphology of myotubes in culture and the expression of key proteins that regulate myogenesis in young and adult rats. Eur J Nutr 50(4):243–250. https://doi.org/10.1007/s00394-010-0132-9
Gam CM, Mortensen OH, Qvortrup K, Damm P, Quistorff B (2015) Effect of high-fat diet on rat myometrium during pregnancy-isolated myometrial mitochondria are not affected. Pflugers Arch 467(7):1539–1549. https://doi.org/10.1007/s00424-014-1599-7
Schrauwen P, Schrauwen-Hinderling V, Hoeks J, Hesselink MK (2010) Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta 1801(3):266–271. https://doi.org/10.1016/j.bbalip.2009.09.011
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435(2):297–312. https://doi.org/10.1042/BJ20110162
Lekva T, Norwitz ER, Aukrust P, Ueland T (2016) Impact of systemic inflammation on the progression of gestational diabetes mellitus. Curr Diab Rep 16(4):26. https://doi.org/10.1007/s11892-016-0715-9
Evangelista AF, Collares CV, Xavier DJ et al. (2014) Integrative analysis of the transcriptome profiles observed in type 1, type 2 and gestational diabetes mellitus reveals the role of inflammation. BMC Med Genom 7:28. https://doi.org/10.1186/1755-8794-7-28
Brochner-Mortensen J, Ditzel J (1982) Glomerular filtration rate and extracellular fluid volume in insulin-dependent patients with diabetes mellitus. Kidney Int 21(5):696–698
Fauchald P, Norseth J, Jervell J (1985) Transcapillary colloid osmotic gradient, plasma volume and interstitial fluid volume in long-term type 1 (insulin-dependent) diabetes. Diabetologia 28(5):269–273
Skyler JS, Bakris GL, Bonifacio E et al. (2017) Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 66(2):241–255. https://doi.org/10.2337/db16-0806
Fernandez-Marcos PJ, Auwerx J (2011) Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93(4):884S-890. https://doi.org/10.3945/ajcn.110.001917
Scarpulla RC (2008) Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88(2):611–638. https://doi.org/10.1152/physrev.00025.2007
Rasmussen UF, Rasmussen HN (2000) Human skeletal muscle mitochondrial capacity. Acta Physiol Scand 168(4):473–480. https://doi.org/10.1046/j.1365-201x.2000.00699.x
Mogensen M, Sahlin K (2005) Mitochondrial efficiency in rat skeletal muscle: influence of respiration rate, substrate and muscle type. Acta Physiol Scand 185(3):229–236. https://doi.org/10.1111/j.1365-201X.2005.01488.x
Hoppel CL, Tandler B, Fujioka H, Riva A (2009) Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol 41(10):1949–1956. https://doi.org/10.1016/j.biocel.2009.05.004
Acknowledgements
We thank Mr. Ib Therkelsen, Panum NMR Center, and Mrs. Bettina Starup Mentz, Section for Cellular and Metabolic Research, for expert technical assistance during the conductance of the experiments. We also thank Ms. Zhila Nikrozi, Core Facility for Integrated Microscopy, and Mrs. Heidi Marie Paulsen, Endocrinology Research Section, for expert technical assistance in section preparation for electron microscopy and light microscopy, respectively. The study is part of Christiane Gam’s Ph.D. project funded by the Faculty of Health and Medical Sciences, University of Copenhagen, and by Ph.D. program of Diabetes and Metabolism, Faculty of Health Sciences, University of Southern Denmark.
Author information
Authors and Affiliations
Contributions
CG, BQ, PD, OM, and LL planned and designed the study. CG, PD, and EM were responsible for subject inclusion. CG acquired the blood and tissue samples. CG conducted the mitochondrial respiratory experiments. CG, KQ, and SP conducted the histological analyses. CG, OM, and LL conducted the biochemical analyses, including gene and protein expression analyses. CG and OM did the statistical analyses. All authors contributed in writing the manuscript and coordination of the correspondence between authors was organized by CG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.The study was approved by the regional ethical committee of Copenhagen, Denmark (Protocol No. H-1-2012-070).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Massimo Federici.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gam, C.M.B.F., Mortensen, O.H., Larsen, L.H. et al. Diabetes, myometrium, and mitochondria in pregnant women at term. Acta Diabetol 55, 999–1010 (2018). https://doi.org/10.1007/s00592-018-1171-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1171-6