Abstract
Aims
Clinical and experimental data suggest that early insulin therapy could reduce lipotoxicity in subjects and animal models with type 2 diabetes mellitus. However, the underlying mechanisms need to be clarified. Sterol regulatory element-binding protein 1c (SREBP-1c), which is negatively regulated by AMP-activated protein kinase (AMPK), plays a critical role in lipotoxicity and insulin resistance in skeletal muscle cells. Here, we investigated the effect and molecular mechanism of insulin intervention on the AMPK/SREBP-1c pathway in skeletal muscle cells with chronic exposure to palmitic acid (PA).
Methods
Male C57BL/6 mice were fed with a high-fat diet for 12 weeks and were then treated with insulin, AMPK inhibitor, or metformin. L6 myotubes incubated with palmitic acid (PA) were treated with insulin or metformin. Dominant-negative AMPKα2 (DN-AMPKα2) lentivirus, AMPKα2 siRNA, or Rho-kinase 1 (ROCK1) siRNA were transfected into PA-treated L6 myotubes.
Results
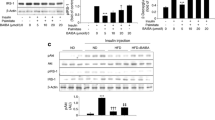
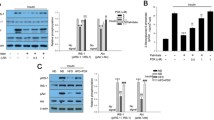
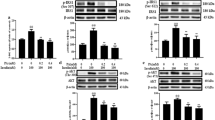
We found that the ability of PA to stimulate SREBP-1c and inhibit AMPK was reversed by insulin in L6 cells. Moreover, DN-AMPKα2 lentivirus and AMPKα2 siRNA were transfected into PA-treated L6 myotubes, and the decrease in SREBP-1c expression caused by insulin was blocked by AMPK inhibition independent of the phosphatidylinositol-4,5-biphosphate-3-kinase (PI3K)/AKT pathway. The serine/threonine kinase Rho-kinase (ROCK) 1, a downstream effector of the small G protein RhoA, was activated by PA. Interestingly, knockdown of ROCK1 by siRNA blocked the downregulation of AMPK phosphorylation under PA-treated L6 myotubes, which indicated that ROCK1 mediated the effect of insulin action on AMPK.
Conclusions
Our study indicated that insulin reduced lipotoxicity via ROCK1 and then improved AMPK/SREBP-1c signaling in skeletal muscle under PA-induced insulin resistance.





Similar content being viewed by others
References
Vaverková H, Chlup R, Ficker L, Novotny D, Bartek J (1997) Complementary insulin therapy improves blood glucose and serum lipid parameters in type 2 (non-insulin-dependent) diabetic patients. II. Effects on serum lipids, lipoproteins and apoproteins. Exp Clin Endocrinol Diabetes 105(Suppl 2):74–77
Hayashi T, Hirano T, Yamamoto T, Ito Y, Adachi M (2006) Intensive insulin therapy reduces small dense low-density lipoprotein particles in patients with type 2 diabetes mellitus: relationship to triglyceride-rich lipoprotein subspecies. Metabolism 55(7):879–884
Juurinen L, Tiikkainen M, Häkkinen AM, Hakkarainen A, Yki-Järvinen H (2007) Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 292(3):E829–E835
Bi Y, Sun WP, Chen X et al (2008) Effect of early insulin therapy on nuclear factor kappaB and cytokine gene expressions in the liver and skeletal muscle of high-fat diet, streptozotocin-treated diabetic rats. Acta Diabetol 45(3):167–178
Sun W, Bi Y, Liang H et al (2011) Inhibition of obesity-induced hepatic ER stress by early insulin therapy in obese diabetic rats. Endocrine 39(3):235–241
Ferré P, Foufelle F (2007) SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 68(2):72–82
Mingrone G, Rosa G, Greco AV et al (2003) Intramyocitic lipid accumulation and SREBP-1c expression are related to insulin resistance and cardiovascular risk in morbid obesity. Atherosclerosis 170(1):155–161
Bi Y, Wu W, Shi J et al (2014) Role for sterol regulatory element binding protein-1c activation in mediating skeletal muscle insulin resistance via repression of rat insulin receptor substrate-1 transcription. Diabetologia 57(3):592–602
Commerford SR, Peng L, Dubé JJ, O’Doherty RM (2004) In vivo regulation of SREBP-1c in skeletal muscle: effects of nutritional status, glucose, insulin, and leptin. Am J Physiol Regul Integr Comp Physiol 287(1):R218–R227
Wu W, Tang S, Shi J et al (2015) Metformin attenuates palmitic acid-induced insulin resistance in L6 cells through the AMP-activated protein kinase/sterol regulatory element-binding protein-1c pathway. Int J Mol Med 35(6):1734–1740
Li Y, Xu S, Mihaylova MM et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13(4):376–388
Chen X, Yu QQ, Zhu YH et al (2010) Insulin therapy stimulates lipid synthesis and improves endocrine functions of adipocytes in dietary obese C57BL/6 mice. Acta Pharmacol Sin 31(3):341–346
Bi Y, Cai M, Liang H et al (2009) Increased carnitine palmitoyl transferase 1 expression and decreased sterol regulatory element-binding protein 1c expression are associated with reduced intramuscular triglyceride accumulation after insulin therapy in high-fat-diet and streptozotocin-induced diabetic rats. Metabolism 58(6):779–786
Kaplan M, Aviram M, Hayek T (2012) Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol Ther 136(2):175–185
Zhou H, Li YJ (2012) Rho kinase inhibitors: potential treatments for diabetes and diabetic complications. Curr Pharm Des 18(20):2964–2973
Kanda T, Wakino S, Homma K et al (2006) Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 20(1):169–171
Lee SH, Huang H, Choi K et al (2014) ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am J Physiol Endocrinol Metab 306(3):E332–E343
Furukawa N, Ongusaha P, Jahng WJ et al (2005) Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2(2):119–129
Kikuchi Y, Yamada M, Imakiire T et al (2007) A Rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J Endocrinol 192(3):595–603
Liu PY, Chen JH, Lin LJ, Liao JK (2007) Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol 49(15):1619–1624
Noda K, Nakajima S, Godo S et al (2014) Rho-kinase inhibition ameliorates metabolic disorders through activation of AMPK pathway in mice. PLoS ONE 9(11):e110446
Noda K, Godo S, Saito H, Tsutsui M, Shimokawa H (2015) Opposing roles of nitric oxide and rho-kinase in lipid metabolism in mice. Tohoku J Exp Med 235(3):171–183
Tang ST, Zhang Q, Tang HQ, et al (2016) Effects of glucagon-like peptide-1 on advanced glycation endproduct-induced aortic endothelial dysfunction in streptozotocin-induced diabetic rats: possible roles of Rho kinase- and AMP kinase-mediated nuclear factor κB signaling pathways. Endocrine 53(1):107–116
Rachek LI (2014) Free fatty acids and skeletal muscle insulin resistance. Prog Mol Biol Transl Sci 121:267–292
Shao W, Espenshade PJ (2012) Expanding roles for SREBP in metabolism. Cell Metab 16(4):414–419
Krycer JR, Sharpe LJ, Luu W, Brown AJ (2010) The AKT-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol Metab 21(5):268–276
Li Y, Xu S, Mihaylova MM et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13(4):376–388
Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB (2012) p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metab 16:104–112
Fujii N, Jessen N, Goodyear LJ (2006) AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291:E867–E877
Hegarty BD, Turner N, Cooney GJ, Kraegen EW (2009) Insulin resistance and fuel homeostasis: the role of AMP-activated protein kinase. Acta Physiol (Oxford, England) 196:129–145
Fujii N et al (2008) Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes 57:2958–2966
Sun J, Yang T, Wang P et al (2014) Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway. Hypertension 63(6):1354–1363
Tao W et al (2015) Lipid-induced Muscle Insulin Resistance Is Mediated by GGPPS via Modulation of the RhoA/Rho Kinase Signaling Pathway. J Biol Chem 290:20086–20097
Kanda T, Wakino S, Homma K et al (2006) Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 20(1):169–171
Begum N, Duddy N, Sandu O, Reinzie J, Ragolia L (2000) Regulation of myosin-bound protein phosphatase by insulin in vascular smooth muscle cells: evaluation of the role of Rho kinase and phosphatidylinositol-3-kinase-dependent signaling pathways. Mol Endocrinol 14(9):1365–1376
Manning BD (2010) Insulin signaling: inositol phosphates get into the AKT. Cell 143:861–863
Begum N, Ragolia L, Rienzie J, McCarthy M, Duddy N (1998) Regulation of mitogen-activated protein kinase phosphatase-1 induction by insulin in vascular smooth muscle cells. Evaluation of the role of the nitric oxide signaling pathway and potential defects in hypertension. J Biol Chem 273:25164–25170
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China Grant Award (81570736, 81270906, 81570737, 81370947, 81500612, 81500630, 81400832, 81300651, 81600632, 81600637), the Project of National Key Clinical Division, the China Postdoctoral Science Foundation (2012M521050), Jiangsu Province’s Key Discipline of Medicine (XK201105), Jiangsu Province’s Key Provincial Talents Program (RC2011011), Jiangsu Key Laboratory for Molecular Medicine (BM2007208), Jiangsu Province’s Project of Standardized Diagnosis and Treatment of Key Diseases (2015604), Jiangsu Postdoctoral Science Foundation, the Key Project of Nanjing Clinical Medical Science, Nanjing Outstanding Youth Fund Projects (JQX13010), Nanjing Science and Technology Development Projects (2013ZD005), and Medical and Health Research Projects of Nanjing Health Bureau (YKK14055, YKK11092), 2016 China Diabetes Young Scientific Talent Research Project.
Author contributions
Yan Bi and Dalong Zhu contributed to the study design, data interpretation, and final approval of the version to be published. Sunyinyan Tang and Wenjun Wu wrote the main manuscript text and contributed to the acquisition of data and approval of the final version. Wenjuan Tang, Zhijuan Ge, and Ting Hong prepared Figures 1–4 and contributed to acquisition of data. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Sunyinyan Tang, Wenjun Wu, Wenjuan Tang, Zhijuan Ge, Ting Hong, Dalong Zhu, and Yan Bi declare that they have no competing financial interests.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of human and animal rights
All animal procedures were performed according to the National Institutes of Health guidelines and approved by the animal care committee of Drum Tower Hospital affiliated with Nanjing University Medical School (Nanjing, China).
Informed consent
Our study is not clinical trial, so there is no informed consent obtained from individual participants included in the study.
Additional information
Managed by Massimo Porta.
The authors Sunyinyan Tang and Wenjun Wu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, S., Wu, W., Tang, W. et al. Suppression of Rho-kinase 1 is responsible for insulin regulation of the AMPK/SREBP-1c pathway in skeletal muscle cells exposed to palmitate. Acta Diabetol 54, 635–644 (2017). https://doi.org/10.1007/s00592-017-0976-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-0976-z