Abstract
Aims
The incidence of cardiac events is increased in diabetic patients even after lipid-lowering therapy. This study aimed to compare the statin-induced changes in the characteristics of vulnerable plaques in patients without diabetes mellitus (DM) and in those with better- or poorly controlled DM, measured by optical coherence tomography (OCT).
Methods
This was a retrospective study of 99 non-culprit lipid-rich plaques from 75 patients who underwent intensive OCT imaging examination. Thirty-four non-diabetic patients were assigned to Group A. According to the average HbA1c level, 22 diabetic patients were assigned to Group B, and 19, to Group C (average HbA1c < 8 and ≥8 %, respectively).
Results
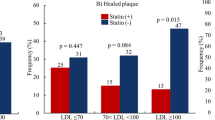
Following 12 months of statin therapy, similar improvements in serum lipid levels were observed in the three groups. However, the increase in fibrous-cap thickness of plaques was the highest in Group A (Group A, 183 %; Group B, 104 %; and Group C, 53 %). Significant reductions in lipid volume index were observed in Groups A and B (Group A: −12 %, P < 0.001; Group B: −13 %, P = 0.038; Group C: 7 %, P = 0.948). Percent changes in OCT measurements were significantly correlated with average HbA1c in patients.
Conclusions
Improved glucose control may enhance the statin-induced reduction in characteristics of vulnerable plaque in diabetic patients.



Similar content being viewed by others
References
Pedersen TR, Kjekshus J, Berg K et al (2004) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Atheroscler Suppl 5(3):81–87
Wei L, Ebrahim S, Bartlett C, Davey PD, Sullivan FM, MacDonald TM (2005) Statin use in the secondary prevention of coronary heart disease in primary care: cohort study and comparison of inclusion and outcome with patients in randomised trials. BMJ 330(7495):821
Sinclair H, Bourantas C, Bagnall A, Mintz GS, Kunadian V (2015) OCT for the identification of vulnerable plaque in acute coronary syndrome. JACC Cardiovasc Imaging 8(2):198–209
Hattori K, Ozaki Y, Ismail TF et al (2012) Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc Imaging 5(2):169–177
Hirayama A, Saito S, Ueda Y et al (2009) Qualitative and quantitative changes in coronary plaque associated with atorvastatin therapy. Circ J 73(4):718–725
Takarada S, Imanishi T, Kubo T et al (2009) Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis 202(2):491–497
Burgmaier M, Hellmich M, Marx N, Reith S (2014) A score to quantify coronary plaque vulnerability in high-risk patients with type 2 diabetes: an optical coherence tomography study. Cardiovasc Diabetol 13:117
Worthley SG, Helft G, Zaman AG, Fuster V, Badimon JJ (2000) Atherosclerosis and the vulnerable plaque–imaging: part II. Aust N Z J Med 30(6):704–710
Yonetsu T, Bouma BE, Kato K, Fujimoto JG, Jang IK (2013) Optical coherence tomography-15 years in cardiology. Circ J 77(8):1933–1940
Wijns W, Shite J, Jones MR et al (2015) Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J 36(47):3346–3355
Jang IK, Bouma BE, Kang DH et al (2002) Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 39(4):604–609
Huang D, Swanson EA, Lin CP et al (1991) Optical coherence tomography. Science 254(5035):1178–1181
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20(5):1262–1275
Quinones A, Lobach I, Maduro GA Jr, Smilowitz NR, Reynolds HR (2015) Diabetes and ischemic heart disease death in people age 25–54: a multiple-cause-of-death analysis based on over 400 000 deaths from 1990 to 2008 in New York City. Clin Cardiol 38(2):114–120
Colayco DC, Niu F, McCombs JS, Cheetham TC (2011) A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 34(1):77–83
Daida H, Takayama T, Hiro T et al (2012) High HbA1c levels correlate with reduced plaque regression during statin treatment in patients with stable coronary artery disease: results of the coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Cardiovasc Diabetol 11:87
Bayturan O, Kapadia S, Nicholls SJ et al (2010) Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol 55(24):2736–2742
Tian J, Dauerman H, Toma C et al (2014) Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol 64(7):672–680
Sato A, Hoshi T, Kakefuda Y et al (2015) In vivo evaluation of fibrous cap thickness by optical coherence tomography for positive remodeling and low-attenuation plaques assessed by computed tomography angiography. Int J Cardiol 182:419–425
Yonetsu T, Kato K, Uemura S et al (2013) Features of coronary plaque in patients with metabolic syndrome and diabetes mellitus assessed by 3-vessel optical coherence tomography. Circ Cardiovasc Imaging 6(5):665–673
Kato K, Yonetsu T, Kim SJ et al (2012) Comparison of nonculprit coronary plaque characteristics between patients with and without diabetes: a 3-vessel optical coherence tomography study. JACC Cardiovasc Interv 5(11):1150–1158
Virmani R, Burke AP, Farb A, Kolodgie FD (2006) Pathology of the vulnerable plaque. J Am Coll Cardiol 47(8 Suppl):C13–C18
Tearney GJ, Yabushita H, Houser SL et al (2003) Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 107(1):113–119
Kataoka Y, Puri R, Hammadah M et al (2014) Spotty calcification and plaque vulnerability in vivo: frequency-domain optical coherence tomography analysis. Cardiovasc Diagn Ther 4(6):460–469
Tian J, Ren X, Uemura S et al (2014) Spatial heterogeneity of neoatherosclerosis and its relationship with neovascularization and adjacent plaque characteristics: optical coherence tomography study. Am Heart J 167(6):884–892
Berry C, Noble S, Gregoire JC et al (2010) Glycaemic status influences the nature and severity of coronary artery disease. Diabetologia 53(4):652–658
Eeg-Olofsson K, Cederholm J, Nilsson PM et al (2010) New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med 268(5):471–482
Zitkus BS (2014) Update on the American Diabetes Association standards of medical care. Nurse Pract 39(8):22–32
Hou J, Xing L, Jia H et al (2016) Comparison of intensive versus moderate lipid-lowering therapy on fibrous cap and atheroma volume of coronary lipid-rich plaque using serial optical coherence tomography and intravascular ultrasound imaging. Am J Cardiol 117(5):800–806
Chia S, Raffel OC, Takano M, Tearney GJ, Bouma BE, Jang IK (2008) Association of statin therapy with reduced coronary plaque rupture: an optical coherence tomography study. Coron Artery Dis 19(4):237–242
Kataoka Y, Puri R, Hammadah M et al (2014) Frequency-domain optical coherence tomographic analysis of plaque microstructures at nonculprit narrowings in patients receiving potent statin therapy. Am J Cardiol 114(4):549–554
Nasu K, Tsuchikane E, Katoh O et al (2008) Plaque characterisation by Virtual Histology intravascular ultrasound analysis in patients with type 2 diabetes. Heart 94(4):429–433
Hong YJ, Jeong MH, Choi YH et al (2009) Plaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patients. JACC Cardiovasc Imaging 2(3):339–349
Matsui T, Oda E, Higashimoto Y, Yamagishi S (2015) Glyceraldehyde-derived pyridinium (GLAP) evokes oxidative stress and inflammatory and thrombogenic reactions in endothelial cells via the interaction with RAGE. Cardiovasc Diabetol 14:1
Vikram A, Tripathi DN, Kumar A, Singh S (2014) Oxidative stress and inflammation in diabetic complications. Int J Endocrinol 2014:679754
Yan SF, Ramasamy R, Schmidt AM (2010) The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res 106(5):842–853
Shiomi M, Ito T, Tsukada T et al (1995) Reduction of serum cholesterol levels alters lesional composition of atherosclerotic plaques. Effect of pravastatin sodium on atherosclerosis in mature WHHL rabbits. Arterioscler Thromb Vasc Biol 15(11):1938–1944
Ishigaki Y, Katagiri H, Gao J et al (2008) Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation 118(1):75–83
Hiro T, Kimura T, Morimoto T et al (2010) Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome-serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN-ACS Trial). Circ J 74(6):1165–1174
Funding
The author Nana Dong has received a Grant from the Open Foundation in China’s Ministry of Education (KF201401) and a Grant from the Graduate Student Innovation Foundation of Heilongjiang Province (YJSCX2012-217HLJ). This study was also supported by National Natural Science Foundation of China (Grant NO.81571749 to J.T. and Grant NO.81330033 to B.Y.), and the National Youth Top-notch Talent Support Program of China (J.T.).
Authors’ contribution
All authors were involved in reporting the results of this study. YB and TJ made substantial contributions to conception, design, and planning of the study. SR, DJ, XZ, and ZY conducted the study and were involved in recruiting patients and keeping the patient database. DN, WW, and SM participated in interpretation of data and statistical analyses. YB and DN wrote the manuscript. YB was responsible for the overall content and served as guarantor. All authors read and approved the final version of the submitted manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical standard
The study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Harbin, China).
Human and Animal Rights
All patients provided written informed consent.
Informed consent
Participants expressed their informed consent for participation in the study.
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, N., Xie, Z., Dai, J. et al. Statin-induced improvements in vulnerable plaques are attenuated in poorly controlled diabetic patients with coronary atherosclerosis disease: a serial optical coherence tomography analysis. Acta Diabetol 53, 999–1008 (2016). https://doi.org/10.1007/s00592-016-0902-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0902-9