Abstract
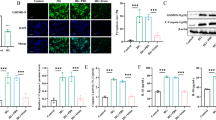
Since most of the current studies of thiazolidinediones (TZDs) are only focused on improving glycemic control, increasing insulin sensitivity, and regulating inflammatory states in Type 2 Diabetes, it is still controversial whether TZDs have direct, protective effects on pancreatic β-cells in autoimmune diabetes. Here, we show the protective effects of TZDs on mouse pancreatic β-cell line cells (NIT-1) impaired by exposure to inflammatory cytokines (IL-1β and IFN-γ) and explore the potential mechanisms for this. The apoptosis rate and caspase-3 activity were remarkably increased, and insulin secretion response to glucose was impaired severely by exposure to IL-1β/IFN-γ for 48 h compared to control cells, whereas apoptosis rate and caspase-3 activity were significantly decreased in cells with treatment of rosiglitazone (RGZ) or pioglitazone (PIG), and the capacity for insulin secretion response to glucose was recovered. TZDs protect pancreatic β-cells from cytokine-induced cytotoxicity through PPARγ activation. The protective effects of the TZDs on NIT-1 cells disappeared when PPARγ was blocked with PPARγ-siRNA interference or treatment with GW9662, the PPARγ antagonist. Additionally, the enhancement of PPARγ expression by treatment with TZDs inhibited the expression of caspase 3 in IL-1β/IFN-γ-induced NIT-cells. Also, the inhibition of caspase 3 expression by TZDs was blocked by co-treatment with GW9662 or infection with PPARγ-siRNA. Taken together, our data suggest that TZDs might serve to protect pancreatic β-cells directly from cytokine-induced cytotoxicity through a PPARγ-dependent pathway, and caspase-3 may play an important role in the mechanisms involved.






Similar content being viewed by others
References
Zhang L, Gianani R, Nakayama M, Liu E, Kobayashi M, Baschal E, Yu L, Babu S, Dawson A, Johnson K, Jahromi M, Aly T, Fain P, Barker J, Rewers M, Eisenbarth GS (2008) Type 1 diabetes: chronic progressive autoimmune disease. Novartis Found Symp 292:85–94
Rossini AA (2004) Autoimmune diabetes and the circle of tolerance. Diabetes 53:267–275
Eizirik DL, Mandrup-Poulsen T (2001) A choice of death: the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia 44:2115–2133
Hui H, Dotta F, Di Mario U, Perfetti R (2004) Role of caspases in the regulation of apoptotic pa ncreatic islet β- cells death. J Cell Physiol 200:177–200
Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P (2000) Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406:739–742
Atz JD, Benoist C, Mathis D (1995) T helper cell subsets in insulin-dependent diabetes. Science 268:1185–1188
Eizirik DL, Darville MI (2001) Beta-cell apoptosis and defense mechanisms: lessons from type 1 diabetes. Diabetes 50(Suppl 1):S64–S69
Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL (2005) Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(Suppl 2):S97–S107
Campbell IL, Kay TW, Oxbrow L, Harrison LC (1991) Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest 87:739–742
Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, Kay TW (2006) Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 116:3258–3265
O’Sullivan BJ, Thomas HE, Pai S, Santamaria P, Iwakura Y, Steptoe RJ, Kay TW, Thomas R (2006) IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol 176:7278–7287
Thomas HE, Irawaty W, Darwiche R, Brodnicki TC, Santamaria P, Allison J, Kay TW (2004) IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes 53:113–121
Wang B, Andre I, Gonzalez A, Katz JD, Aguet M, Benoist C, Mathis D (1997) Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci USA 94:13844–13849
Ortis F, Pirot P, Naamane N, Kreins AY, Rasschaert J, Moore F, Théâtre E, Verhaeghe C, Magnusson NE, Chariot A, Orntoft TF, Eizirik DL (2008) Induction of nuclear factor-κB and its downstream genes by TNF-α and IL-1β has a pro-apoptotic role in pancreatic beta cells. Diabetologia 51:1213–1225
Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS (2007) NF-κB prevents beta cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci USA 104:1913–1918
Philchenkov A (2004) Caspases: potential targets for regulating cell death. J Cell Mol Med 8:432–444
Zhang B, Lu Y, Campbell-Thompson M (2007) α1-antitrypsin protects β-cells from apoptosis. Diabetes 56:1316–1323
Krammer PH (1999) CD95 (Apo-1/Fas)-mediated apoptosis: live and let die. Adv Immunol 71:163–210
Krammer PH (2000) CD95’s deadly mission in the immune system. Nature 407:789–795
Odom J, Williamson B, Carter L (2008) Rosiglitazone and pioglitazone in the treatment of diabetes mellitus. Am J Health-Syst Pharm 65:1846–1850
Villacorta L, Schopfer FJ, Zhang J, Freeman BA, Chen YE (2009) PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond) 116:205–218
Hazra S, Batra RK, Tai HH (2007) Pioglitazone and rosiglitazone decrease pros- taglandin E2 in non-small-cell lung cancer cells by up-regulating 15-hydroxy- prostaglandin dehydrogenase. Mol Pharmacol 71:1715–1720
Artwohl M, Fürnsinn C, Waldhäusl W (2005) Thiazolidinediones inhibit proliferation of microvascular and macrovascular cells by a PPARgamma-independent mechanism. Diabetologia 48:586–594
Gardner OS, Shiau CW, Chen CS (2005) Peroxisome proliferator- activated receptor gamma-independent activation of p38 MAPK by thiazolidinediones involves cal- cium/calmodulin-dependent protein kinase II and protein kinase R: correlation with endoplasmic reticulum stress. J Biol Chem 280:10109–10118
Wei S, Yang J, Lee SL, Kulp SK, Chen CS (2009) PPARgamma-independent antitumor effects of thiazolidinediones. Cancer Lett 276:119–124
Xiong X, Ye Y, Fu L, Dai B, Liu J, Jia J, Tang J, Li L, Wang L, Shen J, Mei C (2009) Antitumor activity of a novel series of alpha-aryloxy-alpha- methylhydrocinnamic acid derivatives as PPAR gamma agonists against a panel of human cancer cell lines. Invest New Drugs 27:223–232
Dewar BJ, Gardner OS, Chen CS, Earp HS, Samet JM, Graves LM (2007) Capacitative calcium entry contributes to the differential transactivation of the epidermal growth factor receptor in response to thiazolidinediones. Mol Pharmacol 72:1146–1156
Weng JR, Chen CY, Pinzone JJ, Ringel MD, Chen CS (2006) Beyond peroxisome proliferator-activated receptor gamma signaling: the multi-facets of the antitumor effect of thiazolidinediones. Endocr Relat Cancer 13:401–413
Tsukamoto H, Hishinuma T, Suzuki N, Tayama R, Hiratsuka M, Yoshihisa T, Mizugaki M, Goto J (2004) Thiazolidinediones increase arachidonic acid release and subsequent prostanoid production in a peroxisome proliferator-activated receptor gamma-independent manner. Prostaglandins Other Lipid Mediat 3:191–213
Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM (2003) Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormal- ities in islet mass without compromising glucose homeostasis. Mol Cell Biol 23:7222–7229
Kim E-K, Kwon K-B, Koo B-S, Han M-J, Song M-Y, Song E-K, Han M-K, Park J-W, Ryu D-G, Park B-H (2007) Activation of peroxisome proliferators-activated receptor-γ protects pancreatic β-cells from cytokine- induced cytotoxicity via NFkB pathway. Int J Biochem Cell Biol 39:1260–1275
Pei J, Zhou Z, Luo J, Jiang T, Li X, He L (2004) Preventive effects of pioglitazone on diabetes and relevant mechanisms, experimental study on non-obese diabetic mice. National Medical Journal of China 84:411–415
Zhou Z, Li X, Huang G, Peng J, Yang L, Yan X, Wang J (2005) Rosiglitazone combined with insulin preserves islet beta cell function in adult-onset latent autoimmune diabetes (LADA). Diabetes Metab Res Rev 21:203–208
Kwon KB, Yang JY, Ryu DG, Rho HW, Kim JS, Park JW, Kim HR, Park BH (2001) Vibrio vulnificus cytolysin induces superoxide anion initiated apoptotic signaling pathway in human ECV304 cells. J Biol Chem 276:47518–47523
Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M (2001) Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 98:10845–10850
Deng WG, Zhu Y, Wu KK (2004) 2004 Role of p300 and PCAF in regulating cyclooxygenase-2 promoter activation by inflammatory mediators. Blood 103:2135–2142
Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H, Chen JJ, Liou JY, Shyue SK, Wu KK (2006) 15d-Prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 26:481–487
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA (1995) Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376:37–43
Park BH, Rho HW, Park JW, Cho CG, Kim JS, Chung HT, Kim HR (1995) Protective mechanism of glucose against alloxan induced pancreatic β-cell damage. Biochem Biophys Res Commun 210:1–6
Bell GI, Polonsky KS (2001) Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 414:788–791
Lin CY, Gurlo T, Haataja L, Hsueh WA, Butler PC (2005) Activation of peroxisome proliferator- activated receptor gamma by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3′-kinase- dependent pathway. J Clin Endocrinol Metab 90:6678–6686
Weber SM, Scarim AL, Corbett JA (2004) PPARγ, is not required for the inhibitory actions of PGJ2 on cytokine signaling in pancreatic cells. Am J Physiol Endocrinol Metab 286:E329–E336
Oshima H, Taketo MM, Oshima M (2006) Destruction of pancreatic β-cells by transgenic induction of prostaglandin E2 in the islets. J Biol Chemistry 281:29330–29336
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic- reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Augstein P, Heinke P, Salzsieder E, Grimm R, Giebel J, Salzsieder C, Harrison LC (2008) Dominance of cytokine-over FasL-induced impairment of the mitochondrial transmembrane potential (Δψm) in the pancreatic β-cell line NIT-1. Diabetes Vasc Dis Res 5:198–204
Pavlovic D, Andersen NA, Mandrup-Poulsen T, Eizirik DL (2000) Activation of extracellular signal-regulated kinase (ERK)1/2 contributes to cytokine-induced apoptosis in purified rat pancreatic β-cells. Eur Cytokine Netw 11:267–274
Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patané G, Boggi U, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P (2002) Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51:1437–1442
Aouad SM, Cohen LY, Sharif-Askari E, Haddad EK, Alam A, Sekaly RP (2004) Caspase-3 is a component of Fas death-inducing signaling complex in lipid rafts and its activity is required for complete caspase-8 activation during Fas-mediated cell death. J Immunol 172:2316–2323
Yamada K, Ichikawa F, Ishiyama SS, Yuan X, Nonaka K (1999) Essential role of caspases-3 in apoptosis of mouse beta-cells transfected with human Fas. Diabetes 48:478–483
Liadis N, Murakami K, Eweida M, Elford AR, Sheu L, Gaisano HY, Hakem R, Ohashi PS, Woo M (2005) Caspase-3-dependent cell apoptosis in the initiation of autoimmune diabetes mellitus. Mol Cell Biol 25:3620–3629
Acknowledgments
This study was supported by grants from National Natural Science Foundation of China (30600298, 30670991), National 973 Project (2006CB503901) and two project of Natural Science Foundation of Hunan province (08JJ4007, 06JJ2086). We would like to thank all the participants in the study. We gratefully acknowledge Prof. Xiao Han (School Of Basic Medical Sciences, Nanjing Medical University, China) for his kindly provide of NIT-1 cells line.
Author information
Authors and Affiliations
Corresponding author
Additional information
An-ping Wang and Xia Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Ap., Li, X., Zheng, Y. et al. Thiazolidinediones protect mouse pancreatic β-cells directly from cytokine-induced cytotoxicity through PPARγ-dependent mechanisms. Acta Diabetol 50, 163–173 (2013). https://doi.org/10.1007/s00592-010-0239-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-010-0239-8