Abstract
Purpose
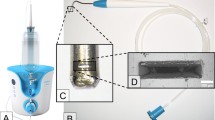
Bacterial biofilms create a challenge in the treatment of prosthetic joint infection (PJI), and failure to eradicate biofilms is often implicated in the high rates of recurrence. In this study, we aimed to compare the effectiveness of a novel nanoparticle ultrasonication technology on Staphylococcus aureus biofilm eradication compared to commonly used orthopedic irrigation solutions.
Methods
Twenty-four sterile, titanium alloy discs were inoculated with a standardized concentration of methicillin-resistant S. aureus and cultured for seven days to allow for biofilm formation. Discs were then treated with either ultrasonicated nanoparticle therapy or irrigation with chlorhexidine gluconate, povidone-iodine or normal saline. The remaining bacteria on each surface was subsequently plated for colony-forming units of S. aureus. Bacterial eradication was reported as a decrease in CFUs relative to the control group. Mann–Whitney U tests were used to compare between groups.
Results
Treatment with ultrasonicated nanoparticles resulted in a significant mean decrease in CFUs of 99.3% compared to controls (p < 0.0001). Irrigation with povidone-iodine also resulted in a significant 77.5% reduction in CFUs compared to controls (p < 0.0001). Comparisons between ultrasonicated nanoparticles and povidone-iodine demonstrated a significantly higher reduction in bacterial CFUs in the nanoparticle group (p < 0.0001).
Conclusion
Ultrasonicated nanoparticle were superior to commonly used bactericidal irrigation solutions in the eradication of S. aureus from a titanium surface. Future clinical studies are warranted to evaluate this ultrsonication technology in the treatment of PJI.


Similar content being viewed by others
References
Ahmed SS, Haddad FS (2019) Prosthetic joint infection. Bone Joint Res 8:570–572. https://doi.org/10.1302/2046-3758.812.BJR-2019-0340
Kelmer G, Stone AH, Turcotte J, King PJ (2021) Reasons for revision: primary total hip arthroplasty mechanisms of failure. J Am Acad Orthop Surg 29:78–87. https://doi.org/10.5435/JAAOS-D-19-00860
Natsuhara KM, Shelton TJ, Meehan JP, Lum ZC (2019) Mortality during total hip periprosthetic joint infection. J Arthroplast 34:S337–S342. https://doi.org/10.1016/j.arth.2018.12.024
Shichman I, Sobba W, Beaton G, Polisetty T, Nguyen HB, Dipane MV et al (2023) The effect of prosthetic joint infection on work status and quality of life: a multicenter. Int Study J Arthroplast 38:2685-2690.e1. https://doi.org/10.1016/j.arth.2023.06.015
Leung F, Richards CJ, Garbuz DS, Masri BA, Duncan CP (2011) Two-stage total hip arthroplasty: how often does it control methicillin-resistant infection? Clin Orthop Relat Res 469:1009–1015. https://doi.org/10.1007/s11999-010-1725-6
Toulson C, Walcott-Sapp S, Hur J, Salvati E, Bostrom M, Brause B et al (2009) Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol. J Arthroplast 24:1051–1060. https://doi.org/10.1016/j.arth.2008.07.004
Bosco F, Cacciola G, Giustra F, Risitano S, Capella M, Vezza D et al (2023) Characterizing recurrent infections after one-stage revision for periprosthetic joint infection of the knee: a systematic review of the literature. Eur J Orthop Surg Traumatol 33:2703–2715. https://doi.org/10.1007/s00590-023-03480-7
Javad Mortazavi SM, Vegari D, Ho A, Zmistowski B, Parvizi J (2011) Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res 469:3049–3054. https://doi.org/10.1007/s11999-011-2030-8
Kubista B, Hartzler RU, Wood CM, Osmon DR, Hanssen AD, Lewallen DG (2012) Reinfection after two-stage revision for periprosthetic infection of total knee arthroplasty. Int Orthop 36:65–71. https://doi.org/10.1007/s00264-011-1267-x
Weinstein EJ, Stephens-Shields AJ, Newcomb CW, Silibovsky R, Nelson CL, O’Donnell JA et al (2023) Incidence, microbiological studies, and factors associated with prosthetic joint infection after total knee arthroplasty. JAMA Netw Open 6:e2340457. https://doi.org/10.1001/jamanetworkopen.2023.40457
Gbejuade HO, Lovering AM, Webb JC (2015) The role of microbial biofilms in prosthetic joint infections. Acta Orthop 86:147–158. https://doi.org/10.3109/17453674.2014.966290
Josse J, Valour F, Maali Y, Diot A, Batailler C, Ferry T et al (2019) Interaction between staphylococcal biofilm and bone: how does the presence of biofilm promote prosthesis loosening. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01602
Soundarrajan D, Rajkumar N, Dhanasekararaja P, Rithika S, Rajasekaran S (2022) A comparison of outcomes of culture positive and culture negative acute knee prosthetic joint infection following debridement, antibiotics and implant retention (DAIR). Eur J Orthop Surg Traumatol 33:2375–2383. https://doi.org/10.1007/s00590-022-03445-2
Lazic I, Scheele C, Pohlig F, von Eisenhart-Rothe R, Suren C (2021) Treatment options in PJI—is two-stage still gold standard? J Orthop 23:180–184. https://doi.org/10.1016/j.jor.2020.12.021
Hameister R, Lim CT, Lohmann CH, Wang W, Singh G (2018) What Is the role of diagnostic and therapeutic sonication in periprosthetic joint infections? J Arthroplast 33:2575–2581. https://doi.org/10.1016/j.arth.2018.02.077
Singh G, Hameister R, Feuerstein B, Awiszus F, Meyer H, Lohmann CH (2014) Low-frequency sonication may alter surface topography of endoprosthetic components and damage articular cartilage without eradicating biofilms completely. J Biomed Mater Res B Appl Biomater 102:1835–1846. https://doi.org/10.1002/jbm.b.33163
Bigelow TA, Northagen T, Hill TM, Sailer FC (2009) The destruction of escherichia coli biofilms using high-intensity focused ultrasound. Ultrasound Med Biol 35:1026–1031. https://doi.org/10.1016/j.ultrasmedbio.2008.12.001
Josic U, Mazzitelli C, Maravic T, Fidler A, Breschi L, Mazzoni A (2022) Biofilm in Endodontics: in vitro cultivation possibilities, sonic-, ultrasonic- and laser assisted removal techniques and evaluation of the cleaning efficacy. Polymers. https://doi.org/10.3390/polym14071334
Welch EC, Chaltas K, Tripathi A (2023) Ultrasound frequency sonication facilitates high-throughput and uniform dissociation of cellular aggregates and tissues. SLAS Technol 28:70–81. https://doi.org/10.1016/j.slast.2023.01.001
Qian Z, Stoodley P, Pitt WG (1996) Effect of low-intensity ultrasound upon biofilm structure from confocal scanning laser microscopy observation. Biomaterials 17:1975–1980. https://doi.org/10.1016/0142-9612(96)00022-1
Piyasena P, Mohareb E, McKellar RC (2003) Inactivation of microbes using ultrasound: a review. Int J Food Microbiol 87:207–216. https://doi.org/10.1016/S0168-1605(03)00075-8
Serena T, Lee SK, Lam K, Attar P, Meneses P, Ennis W (2009) The impact of noncontact, nonthermal, low-frequency ultrasound on bacterial counts in experimental and chronic wounds. Ostomy Wound Manage 55:22–30
Seth AK, Nguyen KT, Geringer MR, Hong SJ, Leung KP, Mustoe TA et al (2013) Noncontact, low-frequency ultrasound as an effective therapy against Pseudomonas aeruginosa–infected biofilm wounds. Wound Repair Regen 21:266–274. https://doi.org/10.1111/wrr.12000
Joyce E, Al-Hashimi A, Mason TJ (2011) Assessing the effect of different ultrasonic frequencies on bacterial viability using flow cytometry. J Appl Microbiol 110:862–870. https://doi.org/10.1111/j.1365-2672.2011.04923.x
Rothenberg AC, Wilson AE, Hayes JP, O’Malley MJ, Klatt BA (2017) Sonication of arthroplasty implants improves accuracy of periprosthetic joint infection cultures. Clin Orthop Relat Res 475:1827–1836. https://doi.org/10.1007/s11999-017-5315-8
Sebastian S, Malhotra R, Sreenivas V, Kapil A, Chaudhry R, Dhawan B (2018) Sonication of orthopaedic implants: a valuable technique for diagnosis of prosthetic joint infections. J Microbiol Methods 146:51–54. https://doi.org/10.1016/j.mimet.2018.01.015
Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME (2011) Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459. https://doi.org/10.4161/viru.2.5.17724
Svensson Malchau K, Tillander J, Zaborowska M, Hoffman M, Lasa I, Thomsen P et al (2021) Biofilm properties in relation to treatment outcome in patients with first-time periprosthetic hip or knee joint infection. J Orthop Translat 30:31–40. https://doi.org/10.1016/j.jot.2021.05.008
Barton CB, Wang DL, An Q, Brown TS, Callaghan JJ, Otero JE (2020) Two-Stage exchange arthroplasty for periprosthetic joint infection following total hip or knee arthroplasty is associated with high attrition rate and mortality. J Arthroplast 35:1384–1389. https://doi.org/10.1016/j.arth.2019.12.005
Zhu MF, Kim K, Cavadino A, Coleman B, Munro JT, Young SW (2021) Success rates of debridement, antibiotics, and implant retention in 230 infected total knee arthroplasties: implications for classification of periprosthetic joint infection. J Arthroplast 36:305–310. https://doi.org/10.1016/j.arth.2020.07.081
Duque AF, Post ZD, Lutz RW, Orozco FR, Pulido SH, Ong AC (2017) Is there still a role for irrigation and debridement with liner exchange in acute periprosthetic total knee infection? J Arthroplast 32:1280–1284. https://doi.org/10.1016/j.arth.2016.10.029
Shohat N, Goh GS, Harrer SL, Brown S (2022) Dilute povidone-iodine irrigation reduces the rate of periprosthetic joint infection following hip and knee arthroplasty: an analysis of 31,331 cases. J Arthroplast 37:226-231.e1. https://doi.org/10.1016/j.arth.2021.10.026
Calkins TE, Culvern C, Nam D, Gerlinger TL, Levine BR, Sporer SM et al (2020) Dilute betadine lavage reduces the risk of acute postoperative periprosthetic joint infection in aseptic revision total knee and hip arthroplasty: a randomized controlled trial. J Arthroplast 35:538-543.e1. https://doi.org/10.1016/j.arth.2019.09.011
Kataoka M, Tsumura H, Kaku N, Torisu T (2006) Toxic effects of povidone–iodine on synovial cell and articular cartilage. Clin Rheumatol 25:632–638. https://doi.org/10.1007/s10067-005-0133-x
von Keudell A, Canseco JA, Gomoll AH (2013) Deleterious effects of diluted povidone-iodine on articular cartilage. J Arthroplast 28:918–921. https://doi.org/10.1016/j.arth.2013.02.018
van Meurs SJ, Gawlitta D, Heemstra KA, Poolman RW, Vogely HC, Kruyt MC (2014) Selection of an optimal antiseptic solution for intraoperative irrigation. J Bone Joint Surg 96:285–291. https://doi.org/10.2106/JBJS.M.00313
Driesman A, Shen M, Feng JE, Waren D, Slover J, Bosco J et al (2020) Perioperative chlorhexidine gluconate wash during joint arthroplasty has equivalent periprosthetic joint infection rates in comparison to betadine wash. J Arthroplast 35:845–848. https://doi.org/10.1016/j.arth.2019.10.009
Lung BE, Le R, Callan K, McLellan M, Issagholian L, Yi J et al (2022) Chlorhexidine gluconate lavage during total joint arthroplasty may improve wound healing compared to dilute betadine. J Exp Orthop 9:67. https://doi.org/10.1186/s40634-022-00503-w
Premkumar A, Nishtala SN, Nguyen JT, Bostrom MPG, Carli AV (2021) The AAHKS best podium presentation research award: comparing the efficacy of irrigation solutions on staphylococcal biofilm formed on arthroplasty surfaces. J Arthroplast 36:S26-32. https://doi.org/10.1016/j.arth.2021.02.033
Tan TL, Kheir MM, Shohat N, Tan DD, Kheir M, Chen C et al (2018) Culture-negative periprosthetic joint infection: an update on what to expect. JB JS Open Access 3:e0060. https://doi.org/10.2106/JBJS.OA.17.00060
Bellova P, Knop-Hammad V, Königshausen M, Mempel E, Frieler S, Gessmann J et al (2019) Sonication of retrieved implants improves sensitivity in the diagnosis of periprosthetic joint infection. BMC Musculoskelet Disord 20:623. https://doi.org/10.1186/s12891-019-3006-1
Tani S, Lepetsos P, Stylianakis A, Vlamis J, Birbas K, Kaklamanos I (2018) Superiority of the sonication method against conventional periprosthetic tissue cultures for diagnosis of prosthetic joint infections. Eur J Orthop Surg Traumatol 28:51–57. https://doi.org/10.1007/s00590-017-2012-y
Kim K, Zhu M, Cavadino A, Munro JT, Young SW (2019) Failed debridement and implant retention does not compromise the success of subsequent staged revision in infected total knee arthroplasty. J Arthroplasty 34:1214-1220.e1. https://doi.org/10.1016/j.arth.2019.01.066
Acknowledgements
Dr. Ran Schwarzkopf an unpaid advisor to Dimoveo Medical. He is a paid consultant for Smith + Nephew and Intellijoint. He receives research support from Smith + Nephew, Osteal and Aerobix as well as royalties from Smith + Nephew related to design. He also has stock options related to Intellijoint, Gauss Surgical and PSI. Yair Ramot is the CEO of Dimoveo Medical and Roi Ramot is the R&D manager of Dimoveo Medical which are directly relevant to this study. The rest of the authors listed have no financial or otherwise relevant disclosures.
Funding
There was no outside funding for this study.
Author information
Authors and Affiliations
Contributions
Roi Ramot and Yair Ramot are cofounders of the startup, Dimoveo Medical™, which developed and is the proprieter for some of the new technology discussed in this manuscript. Ran Schwarzkopf is an unpaid consultant for Dimoveo Medical ™. Dimoveo donated samples of the technology for in vitro testing, however, did not supply additional funding. No other authors have disclosures relevant to the current manuscript and topic.
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose the following conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schaffler, B.C., Longwell, M., Byers, B. et al. Nanoparticle ultrasonication outperforms conventional irrigation solutions in eradicating Staphylococcus aureus biofilm from titanium surfaces: an in vitro study. Eur J Orthop Surg Traumatol (2024). https://doi.org/10.1007/s00590-024-03982-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00590-024-03982-y