Abstract
Introduction
It is still unknown whether the creation of blood-free surfaces by the use of tourniquet during total knee arthroplasty (TKA) has an influence on cement penetration and on implant fixation. The aim of this study is to evaluate the cement mantle under tibial component and the occurrence of progressive radiolucent lines (RLLs) according to the use of tourniquet in primary TKA.
Materials and methods
Fifty patients undergone TKA without the use of tourniquet (group 1) were well matched regarding baseline characteristics with 50 TKAs with the use of tourniquet (group 2). Patients were followed up prospectively. Cement mantle thickness was measured using immediate postoperative X-rays, and the occurrence of progressive radiolucency was finally evaluated in 3-year follow-up. New Knee Society Score (KSS) was used to compare clinical outcome between groups.
Results
Mean cement mantle thickness was 9.27 ± 1.86 mm in group 1 versus 10.49 ± 2.31 mm in group 2 (p = 0.005). Mean cumulated width of RLLs in anterioposterior (AP) view was 7.74 ± 6.68 mm in group 1 versus 3.48 ± 4.69 mm in group 2 (p < 0.001). The percentage of RLLs in AP view was related to the cumulated cement mantle thickness in the same view (r = − 0.218, p < 0.05). There was no significant difference between groups at the final follow-up in terms of ROM and new KSS.
Conclusion
Our results suggest that the use of tourniquet increased the cement mantle thickness under tibial implant and had an influence on the occurrence of RLLs in cement–bone interface, which is related to implant survivorship, with this implant design.
Similar content being viewed by others
Introduction
Long-term mechanical fixation of the implants is essential for a successful total knee arthroplasty (TKA). Cement mantle of 3–5 mm between tibial implant and trabecular bone of the tibia is described as optimum to avoid peripheral osteolysis that is associated with loosening [1]. There are many factors that can affect penetration of the bone cement. Specific bone preparation [2], use of cement gun or hand packing [3,4,5] and time of application of cement after mixing [6, 7] are some of them.
Despite all of these modern cementing techniques, aseptic loosening is still the most common indication for revision surgery [8,9,10,11,12,13,14,15]. It is commonly accepted that the etiology of loosening is multifactorial [16]. One of the factors that can easily affect the cementation during TKA is the use of tourniquet during surgery [17]. Firstly, the application of tourniquet offers better visualization due to bloodless field, which would facilitate cementing quality [18], and secondly there is some evidence that the creation of blood-free and dry surfaces may have an influence on cement penetration [17]. However, it is still unknown whether this influence on cement mantle thickness affects implant stability.
In addition, cementing technique and preparation of the bone have an influence on the occurrence of radiolucency in cement–bone interval during immediate postoperative period. [7, 19,20,21,22,23]. Although these radiolucent lines (RLLs) have shown that they have no correlation with clinical outcome [21], neither with the revision rate [22, 23], there are also progressive RLLs, which are commonly associated with early failure [20].
The aim of this study is to examine (1) whether the use of tourniquet during total knee arthroplasty has an influence on cement mantle under tibial prosthesis and (2) whether this phenomenon is related to implant stability, evaluating the progressive radiolucency in 3–year follow-up.
Materials and methods
Study design and participants
This is a retrospective comparative clinical study based on prospectively collected data. The study was approved by the Ethics Committee at our institution (ID number: 80/27-01-2017), and written informed consent was obtained from all participants.
We reviewed our department registry of patients undergone primary total knee arthroplasty (TKA) for end-stage osteoarthritis between January 2014 and December 2014. Our joint registry is prospectively collected data of all patients who undergo joint replacement surgery. The new Knee Society Scoring System (KSS) [24] and Kellgren–Lawrence’s radiographic grading system [25] are administrated during clinical evaluation preoperatively and during follow-up.
One hundred fifty-six primary TKAs in 149 patients from the same surgical team (authors C.M and A.C), using the same implant (MultigGen Plus CR, Lima Corporate, IT) have been found. Data concerning patients’ demographics, perioperative, postoperative and regular follow-up details were collected from patient’s records. In 64 TKAs, tourniquet has not been used during surgery, mainly due to vascular concerns. Six patients were excluded because they received anticoagulation prior to surgery, two cases because of the use of metaphysical stems and two patients because of thromboembolic history. Two patients were lost to follow-up. One patient declined participation, and in one patient’s immediate postoperative X-ray, there was a radiolucent line in zone 1 in anterioposterior view, suggesting bad poor cementation technique, and so he was excluded from the study, as well.
Finally, a group of 50 patients has been formed (group 1), without the use of tourniquet. All patients from group 1 were well matched, one by one case, for baseline characteristics [sex, age, body mass index (BMI), stage of osteoarthritis] with 50 patients (group 2) who underwent TKA with the use of tourniquet, during the same period, by the same surgeons. For instance, if there was a 62-year-old female patient, with 32 kg/m2 BMI and Kellgren stage 3 osteoarthritis in the non-tourniquet group, there should be a female patient, 62 ± 1 SD years old, with 32 kb/m2 ± 1 SD and Kellgren stage 3 osteoarthritis in the second group, from our database. If there was more than one patient with the same characteristics in our registry, a patient has been chosen randomly, using a computer-based randomization process. In the case that there were not any cases matching the demographic characteristics, our criteria were widened to ± 2 SD for age and BMI.
All patients’ were evaluated for progressive radiolucency during common follow-up with the final point at 3 years postoperatively with KSS and knee radiographs.
Surgical technique
All participants received a hybrid cruciate-retaining TKA (MultiGen Plus CR, Lima Corporate, IT) with fixed bearing design, without patella resurfacing. A total amount of 20 g of bone–cement with 250 mg gentamicin (Palacos R+G®, Heraeus, Hanau, Germany) was used with fourth-generation technique (pulsatile lavage, vacuum mixture of the cement, cement gun pressurization) on the trabecular bone of the tibia, only on the surface and not to the stem to fix the tibial component and was pressed for 12 min always with the same pressure, by keeping the knee in full extension with a trail polyethylene, which was the smallest size available, that was allowing full range of motion and was keeping the knee balanced in coronal plane. In cases that tourniquet has been used, a pneumatic tourniquet was applied prior to surgery at the thigh and was inflated at 350 mmHg pressure before skin incision and after the use of Esmarch bandage for exsanguination of the leg, until skin closure. Intramedullary alignment of the tibial implant was used in all cases. One gram of tranexamic acid was administered intravenously prior to surgery, before application of tourniquet. Two intra-articular drainages were applied in every case, which were removed after 24 h. All patients received the same pain management medication and the same physiotherapeutic protocol until discharge.
Radiological evaluation
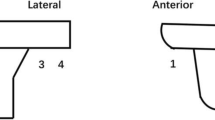
On second postoperative day, supine anterior–posterior (AP) and lateral (Lat) view X-rays were performed under fluoroscopic positioning, in order to place the X-ray beam parallel to the surface of the tibial component. Two authors–evaluators, blinded to the use of tourniquet, evaluated each X-ray to measure the cement mantle thickness under the tibial component, using the institute’s digital Picture Archiving and Communication System (AGFA Impax Client, AGFA Healthcare, Mortsel, Belgium). Measurements were performed twice, with a week interval in order to evaluate the intraobserver’s reliability. Measurements were performed on six zones according to Knee Society Roentgenographic Evaluation System (KSRES) [26], as described by Kopec et al. [4] (Fig. 1). Data were calibrated to the true height of the tibial tray that was 6.3 mm. Because of the random distribution of bone cement that is affected from local bone architecture, measurements were cumulated and the summary was compared between groups.
At 3-year follow-up, the same protocol has been used in order to measure progressive radiolucent lines (RLLs) between cement and bone, which were found during previous follow-up (3-month, 6-month, 1-year, 2-year). Measurements were also performed by the same two authors, in seven AP zones and three Lat zones according to KSRES. Cumulated measurements in each view were used in statistical analysis. Because of the reported poor reliability of KSRES in evaluation of radiolucent lines [27, 28], we also used a suggested simplified system of percentage of radiolucency at the tibial bone–cement interface [29] in our analysis.
Statistical analysis
Based on results of the cumulative mantle thickness under the tibial implant in both groups, for 50 cases in each group and type I error alpha = 0.05, the post hoc statistical power was calculated as 82.9%. In addition, based on the percentage of radiolucency in anteroposterior view, post hoc statistical power was 96.1%, while it was only 46% based on the lateral view. All power analysis results were calculated using a statistical power calculator [30].
Kolmogorov–Smirnov normality test was used and found that data were normally distributed and presented as means and standard deviations. Intraobserver reliability and interobserver reliability were tested by calculation of interclass correlation coefficients (ICC). Parametric tests were used to compare cement mantle thickness, RLLs and % of radiolucency between groups and to correlate the cement mantle thickness with the total summary of RLLs and % of radiolucency under tibial implant. Statistical program was used (SPSS®, ver18; IBM Corp; Somers, NY, USA).
Results
Study sample includes 16 men and 84 women, with mean age of 70.33 ± 6.71 years (range 52–84). Groups were comparable with regard to age, gender, body mass index, stage of osteoarthritis, total range of motion and all subcategories of new Knee Society Score (Table 1).
The correlation coefficient for intraobserver reliability was 0.95 [95% confidence interval (CI) 0.90–0.96] and 0.96 (95% CI 0.90–0.97) for each observer, respectively. The correlation of interobserver reliability was 0.92 (95% CI 0.88–0.97). The cumulated cement mantle thickness was 10.49 ± 2.31 mm for the tourniquet group and 9.27 ± 1.86 mm for the nontourniquet group (p = 0.005) (Fig. 2).
Boxplot of cumulated cement mantle thickness in both types of surgery (with and without tourniquet). Horizontal thick lines are medians. Boxes are formed by the upper and lower quartile. Lower whiskers are 1.5 times the inter-quartile range below the minimum value. Upper whiskers are calculated using the same approach. One outlier is displayed in nontourniquet group
There were no failure/revisions in either group, until final 3-year follow-up.
Clinical and radiological outcomes in both groups are presented in Table 2. There was a significant improvement on all KSS subscales at 3-year follow-up in comparison with the preoperative values (p < 0.001).
The difference between the two groups at the final follow-up was not significantly different in terms of ROM: 99.51 ± 8.14° and 99.69 ± 8.65°, p = 0.916, KSS-objectives: 56.57 ± 4.92 and 56.81 ± 7.02, p = 0.841, KSS-symptoms: 19.43 ± 1.74 and 19 ± 2.12, p = 0.270, KSS-satisfaction: 35.57 ± 5.48 and 34.65 ± 5.82, p = 0.473, KSS-expectation: 9.04 ± 1.64 and 9.08 ± 1.46, p = 0.888, KSS-function 66.04 ± 15.62 and 64.08 ± 16.06, p = 0.540).
Radiographs revealed that 12 of 50 patients of tourniquet group and 25 of 50 patients in nontourniquet group had RLLs in cement–bone interval under tibial plateau in 3-year evaluation.
In the AP view, zone 1 and zone 4 were the common sites for RLLs in both groups. In zone 1, the width of RLLs was significantly higher in nontourniquet group (2.72 ± 2.88 mm than 1.36 ± 1.91 mm, p = 0.007). In zone 4, the width of RLLs was significantly higher in nontourniquet group (2.67 ± 3.27 mm than 1.14 ± 2.32 mm, p = 0.008). There were no significant differences in zones 2, 3, 5, 6 and 7 between groups. In addition, in zone 1, cement mantle thickness was related to the width of RLL (r = − 0.271, p = 0.007). In lateral view, zone 2 was the commonest site of RLLs. There were no significant differences in zones 1, 2 and 3 between groups.
Cumulated width of RLLs in AP view was 3.48 ± 4.69 mm in tourniquet group and 7.74 ± 6.68 mm in nontourniquet group (p < 0.001). Cumulated width of RLLs in lateral view was 1.76 ± 3.42 in tourniquet group and 3.05 ± 4.23 mm in nontourniquet group (p = 0.094). Calibrating the results with the size of the tibial prosthesis, percentage of radiolucency in AP view was 0.05 ± 0.07 in tourniquet group and 0.11 ± 0.09 in nontourniquet group (p < 0.001), and 0.04 ± 0.07 than 0.07 ± 0.09, respectively, in lateral view (p = 0.091). In addition, the percentage of RLLs in AP view had shown a significant but small relationship with the cumulated cement mantle thickness in the same view (r = − 0.218, p = 0.031) (Fig. 3).
Discussion
The main finding of this study is that the use of tourniquet increased the cement mantle thickness under tibial component in primary TKA. This even small increase of the thickness has an influence on the occurrence of progressive RLLs in cement–bone interface at 3-year follow-up. It seems that this is the first study that evaluates the influence of tourniquet on cement mantle and implant stability in mid-term follow-up of 3-years, measuring radiological signs of potential loosening.
The use of tourniquet seems to be a routine during TKA. Ninety-five percent of the members of American Association of Hip and Knee Surgeons use tourniquet during TKA, according to a reported survey [31]. Tourniquet can enhance surgeon’s visualization during surgery and has an influence on reduced total blood loss in cooperation with other techniques (minimal invasive surgery, tranexamic acid), but there is an argument whether this reduced bleeding surface could benefit cement penetration and affect implant fixation [32]. Outcomes from high-level studies about the long-term survivorship without the use of tourniquet are also needed [32]. In contrast, loosening is mostly localized at bone–cement structure [2] and therefore preparation of the bone before cementation is the most important step [19]. It has been proven that suction technique of the trabecular bone improves cement penetration under tibial component [33]. Tourniquet also creates clean and blood-free trabecular bone during TKA, and it may have the same outcome like the suction technique.
Moreover, Pfitzner et al. [17] prospectively compared the cement mantle thickness under tibial component according to the use of tourniquet. They found significant increased thickness in tourniquet group (p = 0.009) but the difference was only 1.2 mm in cumulated thickness of all KSRES zones. Two main concerns have arisen for this study. Firstly, there is little evidence available if this increased thickness of the cement could affect implant stability [7, 34, 35], and secondly, if that increase of only 1 mm could improve implant survivorship. In our study, differences were also significant and small (10.49 ± 2.31 mm in group 1 and 9.27 ± 1.86 mm in group 2, p = 0.005).
The evidence whether the use of tourniquet has a beneficial effect on implant fixation is limited. There are only two studies [36, 37], which investigated the influence of tourniquet on potential implant survivorship using radiostereometric analysis (RSA) and found that there were no differences between the groups. On the other hand, the same authors referred to this technique as a major limitation of their study due to the belief that RSA capability to predict loosening is based only theoretically [36]. Moreover, Vandenbussche et al. [38] found no differences between groups evaluating the radiological signs of loosening in 3-month follow-up. Our main argument is that radiolucent lines, which are the main radiological signs of loosening, cannot be evaluated on short follow-up, because there are also RLLs in immediate postoperative X-rays, which are results of poor cementation technique. These RLLs are non-progressive and they do not affect the fixation of the implant, although they can facilitate the entry of debris to the cement–bone interface [23]. In our patient selection, there was one case in non-tourniquet group that has RLLs on immediate postoperative AP view and was excluded from the study.
Smith et al. [23] also described another type of RLLs that is progressive and can quickly become area of osteolysis, due to the development of fibrous tissue film [39]. These RLLs in the cement–bone interface correlate with migration of the components, loosening [19] and transient bone loss [40], which may be continued over the long term [41]. The analysis of this radiolucency became a common practice during postoperative evaluation of TKA [42]. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (KSRES) uses X-rays to measure the width of RLLs in mm under femoral, tibial and patellar implants in specific areas, which are called zones [26]. The cumulated final width reflects the final score of each implant. The benefit of this method is that it can be a standard way to report outcomes and to compare results. However, there is a consensus about the significance of the scoring of this technique and other more simple methods have been introduced [28, 29]. In our study, in addition of the cumulated width of radiolucent lines and KSRES scoring system, we also used the Chalmers method [29], in which the percentage of radiolucency was used to compare groups. Authors found that 25% or more of the interface was highly predictive of tibial loosening intraoperatively. We found similar significant results using the two methods (KSRES cumulated width and percentage of radiolucency) in both X-ray views.
Although this is a comparative study, its limitations include the fact that it is not a randomized clinical trial and the number of cases is relatively small. The power of this study is that in all cases, done by the same surgeons, radiological evaluation was blinded and the follow-up was prospective. The clinical relevance of this small difference in thickness of cement mantle was also evaluated measuring the radiological outcomes that could predict survival, according to the already existing literature. Further prospective studies with long-term results are necessary to confirm our potential outcome that tourniquet has an influence on implant survival in TKA.
Conclusion
The use of tourniquet during TKA increases cement mantle thickness and reduces the occurrence of radiolucent lines under tibial component, with this implant design. These results could have an influence on long-term implant survivorship.
References
Dorr LD, Lindberg JP, Claude-Faugere M et al (1984) Factors influencing the intrusion of methylmethacrylate into human tibiae. Clin Orthop Relat Res 183:147–152
Maistrelli GL, Antonelli L, Fornasier V, Mahomed N (1995) Cement penetration with pulsed lavage versus syringe irrigation in total knee arthroplasty. Clin Orthop Relat Res 312:261–265
Vanlommel J, Luyckx JP, Labey L et al (2011) Cementing the tibial component in total knee arthroplasty: which technique is the best? J Arthroplasty 26:492–496
Kopec M, Milbrandt JC, Duellman T, Mangan D, Allan DG (2009) Effect of hand packing versus cement gun pressurization on cement mantle in total knee arthroplasty. Can J Surg 52:490–494
Bauze AJ, Costi JJ, Stavrou P et al (2004) Cement penetration and stiffness of the cement-bone composite in the proximal tibia in a porcine model. J Orthop Surg 12:194–198
Krause WR, Krug W, Miller J (1982) Strength of the cement-bone interface. Clin Orthop Relat Res 163:290–299
Walker PS, Soudry M, Ewald FC, McVickar H (1984) Control of cement penetration in total knee arthroplasty. Clin Orthop Relat Res 185:155–164
Aggarwal VK, Goyal N, Deirmengian G, Rangavajulla A, Parvizi J, Austin MS (2014) Revision total knee arthroplasty in the young patient: is there trouble on the horizon? J Bone Joint Surg Am 96:536–542
Arsoy D, Pagnano MW, Lewallen DG, Hanssen AD, Sierra RJ (2013) Aseptic tibial debonding as a cause of early failure in a modern total knee arthroplasty design. Clin Orthop Relat Res 471:94–101
Dalury DF, Pomeroy DL, Gorab RS, Adams MJ (2013) Why are total knee arthroplasties being revised? J Arthroplasty 28:120–121
Hazelwood KJ, O’Rourke M, Stamos VP, McMillan RD, Beigler D, Robb WJ (2015) Case series report: early cement-implant interface fixation failure in total knee replacement. Knee 22:424–428
Lombardi AV Jr., Berend KR, Adams JB (2014) Why knee replacements fail in 2013: patient, surgeon, or implant? Bone Joint J 96-B(11 Suppl A):101–104
Schroer WC, Berend KR, Lombardi AV, Barnes CL, Bolognesi MP, Berend ME et al (2013) Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty 28:116–119
Thiele K, Perka C, Matziolis G, Mayr HO, Sostheim M, Hube R (2015) Current failure mechanisms after knee arthroplasty have changed: polyethylene wear is less common in revision surgery. J Bone Joint Surg Am 97:715–720
Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ (2006) The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop Relat Res 452:28–34
Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C (2006) Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop 77:177–197
Pfitzner T, von Roth P, Voerkelius N, Mayr H, Perka C, Hube R (2016) Influence of the tourniquet on tibial cement mantle thickness in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 24(1):96–101
Tai TW, Lin CJ, Jou IM, Chang CW, Lai KA, Yang CY (2011) Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 7(19):1121–1130
Ritter MA, Herbst SA, Keating EM, Faris PM (1994) Radiolucency at the bone-cement interface in TKR. J Bone Joint Surg Am 76:60–65
Schneider R, Hood RW, Ranawat CS (1982) Radiologic evaluation of knee arthroplasty. Orthop Clin North Am 13:225–244
Ecker ML, Lotke PA, Windsor RE, Cella JP (1987) Long-term results after total condylar knee arthroplasty: significance of radiolucent lines. Clin Orthop Relat Res 216:151–158
Hofmann AA, Goldberg TD, Tanner AM, Cook TM (2006) Surface cementation of stemmed tibial components in primary total knee arthroplasty: minimum 5-year follow-up. J Arthroplasty 21:353–357
Smith S, Naima VS, Freeman MA (1999) The natural history of tibial radiolucent lines in a proximally cemented stemmed total knee arthroplasty. J Arthroplasty 14:3–8
Scuderi GR, Bourne RB, Noble PC, Benjamin JB, Lonner JH, Scott WN (2012) The new knee society knee scoring system. Clin Orthop Relat Res 470:3–19
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis 16:494
Ewald FC (1989) The knee society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res 248:9–12
Bach CM, Steingruber IE, Peer S, Nogler M, Wimmer C, Ogon M (2001) Radiographic assessment in total knee arthroplasty. Clin Orthop Relat Res 385:144–150
Bach CM, Biedermann R, Goebel G, Mayer E, Rachbauer F (2005) Reproducible assessment of radiolucent lines in total knee arthroplasty. Clin Orthop Relat Res 434:183–188
Chalmers BP, Sculco PK, Fehring KA, Trousdale RT, Taunton MJ (2017) A novel Percentage-Based system for determining aseptic loosening of total knee arthroplasty tibial components. J Arthroplasty 32:2274–2278
Rosner B (2011) Fundamentals of biostatistics, 7th edn. Brooks/Cole, Boston
Berry DJ, Bozic KJ (2010) Current practice patterns in primary hip and knee arthroplasty among members of the American association of hip and knee surgeons. J Arthroplasty 25(6 Suppl):2–4
Alcelik I, Pollock RD, Sukeik M, Bettany-Saltikov J, Armstrong PM, Fismer PA (2011) Comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty 27(3):331–340
Stannage K, Shakespeare D, Bulsara M (2003) Suction technique to improve cement penetration under the tibial component in total knee arthroplasty. Knee 10(1):67–73
Bert JM, McShane M (1998) Is it necessary to cement the tibial stem in cemented total knee arthroplasty? Clin Orthop Relat Res 356:73–78
Peters CL, Craig MA, Mohr RA, Bachus KN (2003) Tibial component fixation with cement: full- versus surface-cementation techniques. Clin Orthop Relat Res 409:158–168
Ledin H, Aspenberg P, Good L (2012) Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion. Acta Orthop 83(5):499–503
Molt M, Harsten A, Toksvig-Larsen S (2014) The effect of tourniquet use on fixation quality in cemented total knee arthroplasty a prospective randomized clinical controlled RSA trial. Knee 21(2):396–401
Vandenbussche E, Duranthon LD, Couturier M, Pidhorz L, Augereau B (2002) The effect of tourniquet use in total knee arthroplasty. Int Orthop 26(5):306–309
Reckling FW, Asher MA, Dillon WL (1977) A longitudinal study of the radiolucent line at the bone-cement interface following total joint-replacement procedures. J Bone Joint Surg 59A:355–358
Li MG, Nilsson KG (2000) Changes in bone mineral density at the proximal tibia after total knee arthroplasty: a 2-year follow-up of 28 knees using dual energy X-ray absorptiometry. J Orthop Res 18:40–47
Lonner JH, Klotz M, Levitz C et al (2001) Changes in bone density after cemented total knee arthroplasty: influence of stem design. J Arthroplasty 16:107–111
Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR (2015) Development of a modern Knee Society Radiographic Evaluation System and methodology for total knee arthroplasty. J Arthroplasty 30:2311e4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Touzopoulos, P., Ververidis, A., Mpogiatzis, C. et al. The use of tourniquet may influence the cement mantle thickness under the tibial implant during total knee arthroplasty. Eur J Orthop Surg Traumatol 29, 869–875 (2019). https://doi.org/10.1007/s00590-019-02369-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-019-02369-8