Abstract
Background
Different cell populations from bone marrow were used in various clinical trials for cardiac diseases during last decade. Four clinical studies are ongoing in our institution and enroll patients with cardiac diseases, coronary disease, type 2 diabetes, and osteoarthritis. The density gradient is used to separate bone marrow mononuclear cells. Joint replacement procedures were associated with significant loss of tissue. Usually, excess tissue as bone marrow, peripheral blood and fat are removed to clean operation site. The aim of this study is to prove whether removed tissue during joint replacement procedure can be considered as a significant source of mononuclear cells.
Methods
Excised tissue obtained during joint replacement procedure was collected by AutoLog system. Bone marrow tissue was collected by iliac crest puncture. Mononuclear cells from both sources were isolated by using Ficoll density gradient centrifugation. Flow cytometry was used to detect mononuclear cell, CD34+ population counts and cell viability. Tissue processing yields between the group of joint replacement and iliac crest puncture group were compared.
Results
Together, 34 bone marrow tissue processings were performed. On average, samples contained 46.31 ± 9.35 ml of bone marrow solution. Average cell yield in final product was 28.64 ± 9.35 × 106 MNCs and 0.77 ± 1.51 × 106 CD34+ population. In case of tissue removed during joint replacement nine processings were performed. On average samples contained 450 ± 157.69 ml of tissue solution. Average cell yield in final product was 76.67 ± 35.42 × 106 MNCs and 1.33 ± 0.97 × 106 CD34+ population.
Conclusions
Tissue processing analysis shows that tissue removed during joint replacement procedure can be assumed as a significant source of mononuclear cells. Methods used for bone marrow-derived mononuclear cell extraction can be applied to the excess tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different cell types were evaluated for their regenerative potential and therapeutic applicability for tissue regeneration. Studies are focusing on the use of cells isolated from bone marrow, peripheral blood, skeletal muscle, adipose tissue and umbilical cord, all of which have shown to improve cardiac function in animal models [1]. Among possible cell sources, autologous bone marrow-derived mononuclear cells (BM–MNCs) and their subpopulations are most extensively tested in clinical trials [2,3,4,5].
The leading and most efficient method of surgical treatment on late osteoarthritis stages is total joint replacements. Joint replacement procedures are related to significant loss of tissue on site of operation. A mixture of tissues collected during procedure consists of red bone marrow, peripheral blood, fat, bone debris and rinsing fluid. Usually, the mixture of excised tissue is removed to waste container during rinsing and cleaning of the operation site. So far, no publically available information is present on possible excised tissue use as a mononuclear tissue source.
One of most frequently raised questions related to autologous mononuclear cell application is: Do cell counts matter? From the perspective of autologous application of bone marrow-derived mononuclear cells, therapy could be limited due to variable cell yields. Cell yields vary between individual patients, and the initial tissue sample was restricted due to the internal lumen of the iliac crest.
Besides this, questions concerning cell transplantation exist, including the exact mechanism of the beneficial effect of cell transplantation, the optimal transport of cells into the target tissue, the type and amount of cells implanted, the timing of transplantation, and the assessment of responsiveness of individual patients to the cell therapy [6].
BM–MNC isolation methods are well established; manual or automated processing is performed to produce cell suspensions for transplantation. Currently, different cell delivery methods are employed in clinical praxis, including intracoronary injection and intramyocardial injection [7]. The questions remaining unanswered yet are an effective dose of transplanted cells and best timing for cell transplantation.
In this study, we compared MNC yields between iliac crest puncture and excised tissue groups.
Materials and methods
Study design
Between 2012 and 2016 the patients with hip and knee osteoarthritis received autologous bone marrow-derived mononuclear cell (BM–MNC) therapy. The bone marrow mononuclear cells were extracted from tissue obtained during hip replacement procedure (study group) and by iliac crest puncture (control group). Extracted cells were released for clinical application. This study aims to compare two sources of tissue used for cell acquisition.
State Central Medical Ethics Committee has approved the clinical study. All patients provided informed consent for the study according to the Declaration of Helsinki, and all patients voluntarily agreed to participate and signed informed consent forms.
The 43 patients who received autologous bone marrow-derived mononuclear cell therapy were included in the study according to the inclusion and exclusion criteria and demographics outlined in Tables 1 and 2.
Excised tissue harvesting during joint replacement procedure
During hip replacement operation, artificial joint substitute is inserted. The bone marrow, peripheral blood and fat are removed to rinse and clean operation site prior to insertion of joint substitute. Excised tissue was harvested by AutoLog autotransfusion system (Medtronic) under general anesthesia. Up to 300 ml of liquid phase tissue was aspirated into pre-rinsed AutoLog hardshell reservoir. Operation site was rinsed periodically with sterile 0.9% NaCl (BBraun) containing heparin (500 U/ml). The total volume of heparin and 0.9% NaCl solution used for rinsing was up to 200 ml per tissue harvesting. Solid tissue and bone debris were removed in the waste reservoir. The tissue sample was shipped at room temperature to the central cell processing laboratory and further processed under good manufacturing practice. In short, aspirate was filtrated through 100-µm cell strainer (BD Biosciences) and mononuclear cells were isolated and enriched by density gradient with the use of Ficoll–Paque Premium (GE Healthcare Ltd.) according to manufacturer’s instructions, with minor protocol modifications.
Tissue processing in both patient groups was performed by two biotechnologists to minimize the risks of faults and ensure minimal processing time. All activities, timing, used reagents, batch numbers, shelf-life and personnel involved were recorded to tissue processing file. Each processing is documented in detail and is coded with a particular identifier to ensure processing traceability. All of the processed tissue was released for a therapeutic application.
Bone marrow harvesting and cell preparation
Bone marrow mononuclear cells were harvested by iliac crest puncture performed under local anesthesia. For adult patients, a 38–45 ml of bone marrow was aspirated into heparin pre-filled syringes (500 U/ml of bone marrow aspirate). The bone marrow aspirate was shipped at room temperature to the central cell processing laboratory and further processed under good manufacturing practice. Aspirate was diluted with sterile 0.9% NaCl (1:5) (BBraun) and filtrated through 100-µm cell strainer (BD Biosciences), and bone marrow-derived mononuclear cells (BM–MNCs) were isolated and enriched by density gradient with the use of Ficoll–Paque Premium (GE Healthcare Ltd.) according to manufacturer’s instructions, with minor protocol modifications.
Flow cytometry
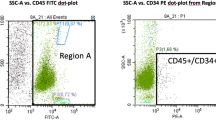
Samples of the final product were taken and used for flow cytometry analysis within 2 h after processing. Stem-Kit from Beckman Coulter was used for cell labeling with CD45-FITC, CD34-PE, 7-AAD and Stem-Count fluorospheres. Cells were analyzed using FC-500 (Beckman Coulter). Analysis protocol was developed manually. StemCXP program was used for MNC count, CD34+ cell count and cell viability detection. Gating was performed according to ISHAGE protocol according to manufacturer’s suggestions. Cell viability was obtained using the 7-AAD method that is included in ISHAGE protocol [8]. Each measurement contained at least 50,000 events. The maximum number of events was 100,000. Obtained numbers of cells/µl were calculated for the total number of MNCs and CD34+ cells within transplantation material. Measurements with less than 50,000 events were excluded from statistical analysis.
Results
Bone marrow-derived mononuclear cells were harvested by iliac crest puncture performed under local anesthesia. On average samples contained 46.31 ml of bone marrow solution. Together, 34 BM tissue processings were performed. Same standard operation procedures for all processings were applied. Each final cell solution was released for clinical application. Final product cell counts were analyzed by flow cytometry (see Table 3).
Tissue removed during joint replacement procedure was harvested by AutoLog system under general anesthesia. Filtrating components of a system designed for blood salvage were removed. Reservoir and suction system was used to collect rinsing solution and tissue. On average samples contained 450 ml of tissue solution. Together, nine excess tissue processings were performed. Same standard operation procedures for all processings were applied. Each final cell solution was released for clinical application. Final product cell counts were analyzed by flow cytometry (see Table 4).
Together, 34 bone marrow tissue processings were performed. On average samples contained 46.31 ± 9.35 ml of bone marrow solution. Average cell yield in final product was 28.64 ± 9.35 × 106 MNCs and 0.77 ± 1.51 × 106 CD34+ population.
In case of tissue removed during joint replacement nine processings were performed. On average samples contained 450 ± 157.69 ml of tissue solution. Average cell yield in final product was 76.67 ± 35.42 × 106 MNCs and 1.33 ± 0.97 × 106 CD34+ population.
Discussion
The isolation of the mononuclear cell fraction is associated with cell loss. Some studies investigating human bone marrow processing show MNC recovery rates between 15 and 30% after Ficoll density gradient centrifugation [9]. Processing has a significant impact on the cell counts, viability and functional activity of bone marrow-derived progenitor cells. The assessment of cell counts and viability may not entirely reflect the functional quality of cells in clinical application. Some groups suggested that controversial clinical effects in large-scale clinical trials are due to technological differences in cell processing and MNC composition [10]. In fact, it has been proven that efficacy and functionality of MNCs are significantly influenced by red blood cell contamination, the content of apoptotic cells, differences in washing steps and even the centrifugation speed. In this study we focus on bone marrow-derived mononuclear cell and excised tissue-derived cell yields after tissue processing, performed using the same methodology for all patients.
Factors that could affect the cell processing outcome associated with bone marrow extraction are as follows: instruments used—aspiration needle specification (side holes, diameter) and aspiration needle placement (depth, angle, radio control used)—the applied negative pressure, administration of anticoagulation agents, peripheral blood volume in the sample, and the individual patient specificities—iliac crest thickness and internal lumens (vary depending on age, gender and health conditions). In case of excised tissue group main challenges were related to administration of anticoagulation agents—rinsing of surfaces with anticoagulating agent solution is essential to minimize clotting that can lead to lower cell counts in the final product. Preliminary trials show that the presence of fat tissue in processing samples can significantly lower cell yields. Increased application of rinsing solution can lead to higher processing losses and expenses related to materials and consumables. Excised tissue group average MNC extraction yield is 2.71 times higher (p = 0.002) comparing to iliac crest puncture group, see Figs. 1 and 2. In case of CD34+ cell fraction statistically significant differences in cell yields were not detected. This finding could be associated with asymmetric processing losses (fat contamination, blood clotting) and/or patient age in excised tissue group.
Conclusions
Tissue processing analysis shows that tissue removed during joint replacement procedure can be assumed as a significant source of mononuclear cells. Methods used for bone marrow-derived mononuclear cell extraction can be applied to the excised tissue. Detailed instructions of harvesting of excised tissue should be developed to minimize fat contamination and minimize volume of rinsing solution in a harvested tissue sample.
Abbreviations
- BM:
-
Bone marrow
- MNC:
-
Mononuclear cell
References
Lachtermacher S, Esporcatte BL, de Azevedo Fortes DS, Rocha NN, Montalvao F et al (2012) Functional and transcriptomic recovery of infracted mouse myocardium treated with bone marrow mononuclear cells. Stem Cell Rev 8:251–261
Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A et al (2002) Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106:1913–1918
Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E (2012) Stemcell treatment for acutemyocardial infarction. Cochrane Database Systemat Rev (2). Art. no.: CD006536
Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R et al (2006) Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 355:1210–1221
Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M (2006) Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 355:1199–1209
Korf-Klingebiel M, Kempf T, Sauer T, Brinkmann E, Fischer P et al (2008) Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur Heart J 29:2851–2858
Leri A, Kajstura J, Anversa P, Frishman WH (2009) Myocardial regeneration and stem cell repair. Curr Probl Cardiol 33:91–153
Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR (1998) Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. Cytometry 34(2):61–70
van Beem RT, Hirsch A, Lommerse IM, Zwaginga JJ, Noort WA, Biemond BJ, Piek JJ, van der Schoot CE, Voermans C (2008) Recovery and functional activity of mononuclear bone marrow and peripheral blood cells after different cell isolation protocols used in clinical trials for cell therapy after acute myocardial infarction. EuroIntervention 4(1):133–138
Seeger Florian H, Tonn Torsten, Krzossok Nicola, Zeiher Andreas M, Dimmeler Stefanie (2007) Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J 28:766–772
Acknowledgements
This study was supported in part by Latvian National Research Program “Biomedicine for Public Health” (BIOMEDICINE) and by grant from corporation “Sistemu Inovacijas.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Informed consent
Particular research involved human participants. Informed consent was obtained from each individual patient.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Eriks, J., Kristaps, E., Liene, P. et al. Tissue evacuated during joint replacement procedure as a source of mononuclear cells. Eur J Orthop Surg Traumatol 28, 457–461 (2018). https://doi.org/10.1007/s00590-017-2067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-017-2067-9