Abstract
Purpose
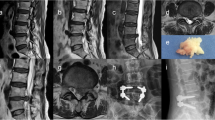
The purpose of this study was to investigate autophagy in an extruded disc and to compare this activity with the activity in the remaining disc after lumbar disc herniation in the same patient.
Methods
In total, 12 patients (females 4, males 8) with the extruded type of lumbar disc herniation (LDH) were surgically treated. Their mean age was 54.3 ± 15.8 years (range: 29 ~ 78 years). The mean interval from the occurrence of symptoms to the operation was 9.8 ± 9.4 weeks (range: 2 ~ 24 weeks). The extruded discs were excised, and the remaining disc material removed, to prevent recurrence of herniation. Immediately after specimen collection, all tissues were stored at −70 °C prior to analysis. Autophagy was assessed immunohistochemically and via Western blotting for Atg5, Atg7, Atg12, Atg12L1, and Beclin-1. And the relationship between autophagy and apoptosis was investigated by correlation analysis of caspase-3 with autophagy proteins.
Results
The expression levels of autophagic markers were significantly increased in the extruded discs compared to the remaining discs within the same patients. The mean expression levels of Atg5, Atg7, Atg12, and Beclin-1 in extruded discs were statistically significantly higher than those in the remaining discs (P < 0.01, P < 0.001, P < 0.01, and P < 0.001 respectively).
Conclusions
The autophagic pathway was more active in extruded disc material than in remaining disc material within the same patient. This may explain spontaneous resorption of the extruded disc after LDH.



Similar content being viewed by others
References
Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JA, Tans JT, Thomeer RT, Koes BW (2007) Surgery versus prolonged conservative treatment for sciatica. N Engl J Med 356:2245–2256. https://doi.org/10.1056/NEJMoa064039
Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, Herkowitz H, Fischgrund J, Cammisa FP, Albert T, Deyo RA (2006) Surgical vs nonoperative treatment for lumbar disk herniation: the spine patient outcomes research trial (SPORT) observational cohort. JAMA 296:2451–2459. https://doi.org/10.1001/jama.296.20.2451
Seo JY, Roh YH, Kim YH, Ha KY (2016) Three-dimensional analysis of volumetric changes in herniated discs of the lumbar spine: does spontaneous resorption of herniated discs always occur? Eur Spine J 25:1393–1402. https://doi.org/10.1007/s00586-014-3587-1
Chiu CC, Chuang TY, Chang KH, Wu CH, Lin PW, Hsu WY (2015) The probability of spontaneous regression of lumbar herniated disc: a systematic review. Clin Rehabil 29:184–195. https://doi.org/10.1177/0269215514540919
Haro H, Kato T, Komori H, Osada M, Shinomiya K (2002) Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J Orthop Res 20:409–415. https://doi.org/10.1016/S0736-0266(01)00150-4
Arai Y, Yasuma T, Shitoto K, Yamauchi Y, Suzuki F (2000) Immunohistological study of intervertebral disc herniation of lumbar spine. J Orthop Sci 5:229–231. https://doi.org/10.1007/s007760000050229.776
Yasuma T, Arai K, Yamauchi Y (1993) The histology of lumbar intervertebral disc herniation. The significance of small blood vessels in the extruded tissue. Spine (Phila Pa 1976) 18:1761–1765
Henmi T, Sairyo K, Nakano S, Kanematsu Y, Kajikawa T, Katoh S, Goel VK (2002) Natural history of extruded lumbar intervertebral disc herniation. J Med Invest 49:40–43
Ha KY, Koh IJ, Kirpalani PA, Kim YY, Cho YK, Khang GS, Han CW (2006) The expression of hypoxia inducible factor-1alpha and apoptosis in herniated discs. Spine (Phila Pa 1976) 31:1309–1313. https://doi.org/10.1097/01.brs.0000219493.76081.d6
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. N Engl J Med 361:1570–1583. https://doi.org/10.1056/NEJMra0901217
Hirata H, Yurube T, Kakutani K, Maeno K, Takada T, Yamamoto J, Kurakawa T, Akisue T, Kuroda R, Kurosaka M, Nishida K (2014) A rat tail temporary static compression model reproduces different stages of intervertebral disc degeneration with decreased notochordal cell phenotype. J Orthop Res 32:455–463. https://doi.org/10.1002/jor.22533
Quan M, Hong MW, Ko MS, Kim YY (2020) Relationships between disc degeneration and autophagy expression in human nucleus pulposus. Orthop Surg 12:312–320. https://doi.org/10.1111/os.12573
Minamide A, Tamaki T, Hashizume H, Yoshida M, Kawakami M, Hayashi N (1998) Effects of steroid and lipopolysaccharide on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine (Phila Pa 1976) 23:870–876. https://doi.org/10.1097/00007632-199804150-00007
Yoshida M, Nakamura T, Sei A, Kikuchi T, Takagi K, Matsukawa A (2005) Intervertebral disc cells produce tumor necrosis factor alpha, interleukin-1beta, and monocyte chemoattractant protein-1 immediately after herniation: an experimental study using a new hernia model. Spine (Phila Pa 1976) 30:55–61. https://doi.org/10.1097/01.brs.0000149194.17891.bf
Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM (2000) Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest 105:143–150. https://doi.org/10.1172/jci7091
Doita M, Kanatani T, Ozaki T, Matsui N, Kurosaka M, Yoshiya S (2001) Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine (Phila Pa 1976) 26:1522–1527. https://doi.org/10.1097/00007632-200107150-00004
Kong CG, Park JB, Kim SH (2023) Inhibitory effect of insulin treatment on apoptosis of intervertebral disc cells in a streptozotocin-induced diabetic rat model. Asian Spine J 17:1–7. https://doi.org/10.31616/asj.2021.0514
Park JB, Chang H, Kim KW (2001) Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine (Phila Pa 1976) 26:618–621. https://doi.org/10.1097/00007632-200103150-00011
Ha KY, Kim BG, Kim KW, Oh IS, Seo JY (2011) Apoptosis in the sequestrated nucleus pulposus compared to the remaining nucleus pulposus in the same patient. Spine (Phila Pa 1976) 36:683–689. https://doi.org/10.1097/BRS.0b013e3181da0286
Zhang L, Niu T, Yang SY, Lu Z, Chen B (2008) The occurrence and regional distribution of DR4 on herniated disc cells: a potential apoptosis pathway in lumbar intervertebral disc. Spine (Phila Pa 1976) 33:422–427. https://doi.org/10.1097/BRS.0b013e318163e036
Olmarker K, Blomquist J, Strömberg J, Nannmark U, Thomsen P, Rydevik B (1995) Inflammatogenic properties of nucleus pulposus. Spine (Phila Pa 1976) 20:665–669. https://doi.org/10.1097/00007632-199503150-00006
Wang J, Tang T, Yang H, Yao X, Chen L, Liu W, Li T (2007) The expression of Fas ligand on normal and stabbed-disc cells in a rabbit model of intervertebral disc degeneration: a possible pathogenesis. J Neurosurg Spine 6:425–430. https://doi.org/10.3171/spi.2007.6.5.425
Kim KH, Lee MS (2014) Autophagy–a key player in cellular and body metabolism. Nat Rev Endocrinol 10:322–337. https://doi.org/10.1038/nrendo.2014.35
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20:1992–2003. https://doi.org/10.1091/mbc.E08-12-1249
Zhang SJ, Yang W, Wang C, He WS, Deng HY, Yan YG, Zhang J, Xiang YX, Wang WJ (2016) Autophagy: a double-edged sword in intervertebral disk degeneration. Clin Chim Acta 457:27–35. https://doi.org/10.1016/j.cca.2016.03.016
Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27:107–132. https://doi.org/10.1146/annurev-cellbio-092910-154005
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752. https://doi.org/10.1038/nrm2239
Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (2013) Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833:3448–3459. https://doi.org/10.1016/j.bbamcr.2013.06.001
Song S, Tan J, Miao Y, Li M, Zhang Q (2017) Crosstalk of autophagy and apoptosis: involvement of the dual role of autophagy under ER stress. J Cell Physiol 232:2977–2984. https://doi.org/10.1002/jcp.25785
Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G (2007) Cell death modalities: classification and pathophysiological implications. Cell Death Differ 14:1237–1243. https://doi.org/10.1038/sj.cdd.4402148
Acknowledgements
None.
Funding
This work was supported by a research grant from Jeju National University Hospital in 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seo, JY., Kim, J., Kim, YY. et al. Autophagy in an extruded disc compared to the remaining disc after lumbar disc herniation in the same patient. Eur Spine J 33, 61–67 (2024). https://doi.org/10.1007/s00586-023-07731-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07731-3