Abstract
Purpose
An osteoporotic vertebral fracture (OVF) is a common disease that causes disabilities in elderly patients. In particular, patients with nonunion following an OVF often experience severe back pain and require surgical intervention. However, nonunion diagnosis generally takes more than six months. Although several studies have advocated the use of magnetic resonance imaging (MRI) observations as predictive factors, they exhibit insufficient accuracy. The purpose of this study was to create a predictive model for OVF nonunion using machine learning (ML).
Methods
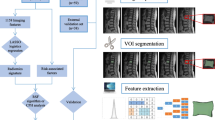
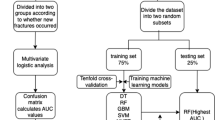
We used datasets from two prospective cohort studies for OVF nonunion prediction based on conservative treatment. Among 573 patients with acute OVFs exceeding 65 years in age enrolled in this study, 505 were analyzed. The demographic data, fracture type, and MRI observations of both studies were analyzed using ML. The ML architecture utilized in this study included a logistic regression model, decision tree, extreme gradient boosting (XGBoost), and random forest (RF). The datasets were processed using Python.
Results
The two ML algorithms, XGBoost and RF, exhibited higher area under the receiver operating characteristic curves (AUCs) than the logistic regression and decision tree models (AUC = 0.860 and 0.845 for RF and XGBoost, respectively). The present study found that MRI findings, anterior height ratio, kyphotic angle, BMI, VAS, age, posterior wall injury, fracture level, and smoking habit ranked as important features in the ML algorithms.
Conclusion
ML-based algorithms might be more effective than conventional methods for nonunion prediction following OVFs.





Similar content being viewed by others
References
Lyles KW, Gold DT, Shipp KM et al (1993) Association of osteoporotic vertebral compression fractures with impaired functional status. Am J Med 94:595–601. https://doi.org/10.1016/0002-9343(93)90210-G
Kado DM, Duong T, Nevitt MC et al (2003) Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int 14:589–594. https://doi.org/10.1007/s00198-003-1412-5
Lips P, van Schoor NM (2005) Quality of life in patients with osteoporosis. Osteoporos Int 16:447–455. https://doi.org/10.1007/S00198-004-1762-7/TABLES/4
Takahashi S, Hoshino M, Takayama K et al (2017) Time course of osteoporotic vertebral fractures by magnetic resonance imaging using a simple classification: a multicenter prospective cohort study. Osteoporos Int 28:473–482. https://doi.org/10.1007/s00198-016-3737-x
Takahashi S, Hoshino M, Takayama K et al (2016) Predicting delayed union in osteoporotic vertebral fractures with consecutive magnetic resonance imaging in the acute phase: a multicenter cohort study. Osteoporos Int 27:3567–3575. https://doi.org/10.1007/s00198-016-3687-3
Ahmadi SA, Takahashi S, Hoshino M et al (2019) Association between MRI findings and back pain after osteoporotic vertebral fractures: a multicenter prospective cohort study. Spine J 19:1186–1193. https://doi.org/10.1016/j.spinee.2019.02.007
Kim DH, Vaccaro AR (2006) Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J 6:479–487. https://doi.org/10.1016/J.SPINEE.2006.04.013
Takahashi S, Hoshino M, Terai H et al (2018) Differences in short-term clinical and radiological outcomes depending on timing of balloon kyphoplasty for painful osteoporotic vertebral fracture. J Orthop Sci 23:51–56. https://doi.org/10.1016/j.jos.2017.09.019
Ha K-Y, Lee J-S, Kim K-W, Chon J-S (2006) Percutaneous vertebroplasty for vertebral compression fractures with and without intravertebral clefts. J Bone Jt Surg Br 88:629–633. https://doi.org/10.1302/0301-620X.88B5.17345
Min H-K, Ahn J-H, Ha K-Y et al (2019) Effects of anti-osteoporosis medications on radiological and clinical results after acute osteoporotic spinal fractures: a retrospective analysis of prospectively designed study. Osteoporos Int 30:2249–2256. https://doi.org/10.1007/s00198-019-05125-0
Iwata A, Kanayama M, Oha F et al (2017) Does spinopelvic alignment affect the union status in thoracolumbar osteoporotic vertebral compression fracture? Eur J Orthop Surg Traumatol 27:87–92. https://doi.org/10.1007/s00590-016-1844-1
Inose H, Kato T, Ichimura S et al (2020) Risk factors of nonunion after acute osteoporotic vertebral fractures: a prospective multicenter cohort study. Spine (Phila Pa 1976) 45:895–902. https://doi.org/10.1097/BRS.0000000000003413
Ha KY, Kim YH (2013) Risk factors affecting progressive collapse of acute osteoporotic spinal fractures. Osteoporos Int 24:1207–1213. https://doi.org/10.1007/s00198-012-2065-z
Jordan MI, Mitchell TM (2015) Machine learning: trends, perspectives, and prospects. Science 349:255–260
Saraswat P (2022) Supervised machine learning algorithm: a review of classification techniques. In: Smart Innovation, Systems and Technologies. pp 477–482
Kourou K, Exarchos TP, Exarchos KP et al (2015) Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J 13:8–17
Chen T, Guestrin C (2016) XGBoost: a scalable tree boosting system. In: Proceedings of the ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. ACM, New York, NY, USA, pp 785–794
Segal MR (2004) Machine learning benchmarks and random forest regression. Biostatistics 1–14
Tsujio T, Nakamura H, Terai H et al (2011) Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion. Spine (Phila Pa 1976) 36:1229–1235. https://doi.org/10.1097/BRS.0b013e3181f29e8d
Kanchiku T, Imajo Y, Suzuki H et al (2013) Usefulness of an early MRI-based classification system for predicting vertebral collapse and pseudoarthrosis after osteoporotic vertebral fractures. J Spinal Disord Tech 27:61–65
Sagi O, Rokach L (2018) Ensemble learning a survey Wiley Interdiscip. Rev Data Min Knowl Discov 8(4):1249
Misra P, Yadav AS (2020) Improving the classification accuracy using recursive feature elimination with cross-validation. Int J Emerg Technol 11:659–665
Gunduz H (2021) An efficient stock market prediction model using hybrid feature reduction method based on variational autoencoders and recursive feature elimination. Financ Innov 7:1–24. https://doi.org/10.1186/s40854-021-00243-3
Darst BF, Malecki KC, Engelman CD (2018) Using recursive feature elimination in random forest to account for correlated variables in high dimensional data. BMC Genet 19:65. https://doi.org/10.1186/s12863-018-0633-8
Lundberg SM, Erion GG, Lee S-I (2018) consistent individualized feature attribution for tree ensembles
Li N, Li B, Gao L (2020) Transient stability assessment of power system based on XGBoost and factorization machine. IEEE Access 8:28403–28414. https://doi.org/10.1109/ACCESS.2020.2969446
Šimundić AM (2011) Measures of diagnostic accuracy. pp 13–55
ElShawi R, Sherif Y, Al-Mallah M, Sakr S (2021) Interpretability in healthcare: a comparative study of local machine learning interpretability techniques. Comput Intell 37:1633–1650. https://doi.org/10.1111/coin.12410
Lee H-C, Yoon S, Yang S-M et al (2018) Prediction of acute kidney injury after liver transplantation: machine learning approaches vs. Logist Regres Model J Clin Med 7:428. https://doi.org/10.3390/jcm7110428
Corradi JP, Thompson S, Mather JF et al (2018) Prediction of incident delirium using a random forest classifier. J Med Syst 42:1–10. https://doi.org/10.1007/S10916-018-1109-0/FIGURES/4
Sheridan RP, Wang WM, Liaw A et al (2016) Extreme gradient boosting as a method for quantitative structure-activity relationships. J Chem Inf Model 56:2353–2360. https://doi.org/10.1021/acs.jcim.6b00591
Ryan G, Magony R, Gortler H et al (2021) Systemically impaired fracture healing in small animal research: a review of fracture repair models. J Orthop Res 39:1359–1367
Goodwin VA, Hall AJ, Rogers E, Bethel A (2016) Orthotics and taping in the management of vertebral fractures in people with osteoporosis: a systematic review. BMJ Open 6:e010657. https://doi.org/10.1136/bmjopen-2015-010657
Kim HJ, Yi JM, Cho HG et al (2014) Comparative study of the treatment outcomes of osteoporotic compression fractures without neurologic injury using a rigid brace, a soft brace, and no brace: a prospective randomized controlled non-inferiority trial. J Bone Jt Surg Am 96:1959–1966. https://doi.org/10.2106/JBJS.N.00187
Kato T, Inose H, Ichimura S et al (2019) Comparison of rigid and soft-brace treatments for acute osteoporotic vertebral compression fracture: a prospective, randomized, multicenter study. J Clin Med 8:198. https://doi.org/10.3390/jcm8020198
Minamide A, Maeda T, Yamada H et al (2018) Early versus delayed kyphoplasty for thoracolumbar osteoporotic vertebral fractures: the effect of timing on clinical and radiographic outcomes and subsequent compression fractures. Clin Neurol Neurosurg 173:176–181. https://doi.org/10.1016/j.clineuro.2018.07.019
Liska F, Haller B, Voss A et al (2018) Smoking and obesity influence the risk of nonunion in lateral opening wedge, closing wedge and torsional distal femoral osteotomies. Knee Surg, Sport Traumatol Arthrosc 26:2551–2557. https://doi.org/10.1007/s00167-017-4754-9
Sloan A, Hussain I, Maqsood M et al (2010) The effects of smoking on fracture healing. Surgeon 8:111–116
Funding
This study was supported by grants from the Health and Labour Sciences Research Grants for Comprehensive Research on Aging and Health, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takahashi, S., Terai, H., Hoshino, M. et al. Machine-learning-based approach for nonunion prediction following osteoporotic vertebral fractures. Eur Spine J 32, 3788–3796 (2023). https://doi.org/10.1007/s00586-022-07431-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07431-4