Abstract
Introduction
Psychological and social factors are involved in the disability and chronicity of pain. Our study aim was to investigate whether social defeat stress (SDS) as a psychophysical stress affected mechanical withdrawal thresholds in the lumbar disk herniation (LDH) rat model. Changes in microglia and astrocytes, which play important roles in neuropathic pain states, were also investigated.
Materials and methods
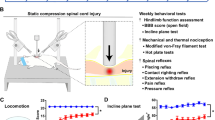
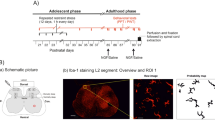
For the LDH model, nucleus pulposus (NP) was applied to the L5 dorsal root ganglion (DRG) in adult female Sprague–Dawley rats. SDS was performed 15 min daily for 8 days. Mechanical withdrawal thresholds were measured, and immunoreactive cells of glial fibrillary acidic protein (GFAP) and ionized calcium-binding adaptor molecule-1 (Iba-1), which were used as markers of microglia, satellite glial cells, and astrocytes, were assessed in the DRG, spinal cord (SC), and ventrolateral periaqueductal gray matter (VLPAG).
Results
Mechanical withdrawal thresholds decreased in the NP group for 21 days and for 35 days in the NP + SDS group. Expression of GFAP and Iba-1 in the DRG and SC increased up to day 21 in the NP and NP + SDS groups. In the sham + SDS and NP + SDS groups, expression of GFAP in the VLPAG decreased until day 35.
Conclusion
SDS prolongs mechanical allodynia induced by NP. Changes of GFAP expression in the VLPAG were associated with mechanical allodynia of the NP + SDS group during the late phase. These results suggest that psychological chronic stress might delay recovery from mechanical allodynia induced by the LDH model.





Similar content being viewed by others
References
Aoki Y, Rydevik B, Kikuchi S, Olmarker K (2002) Local application of disc-related cytokines on spinal nerve roots. Spine (Phila Pa 1976) 27:1614–1617
Arora V, Martin TJ, Aschenbrenner CA, Hayashida K, Kim SA, Parker RA, Eisenach JC, Peter CM (2018) Psychosocial stress delays recovery of postoperative pain following incisional surgery in the rat. Neuroscience 382:35–47
Bair MJ, Robinson RL, Katon W, Kroenke K (2003) Depression and pain comorbidity. Arch Intern Med 163:2433
Bandler R, Shipley MT (1994) Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 17:379–389
Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel S (1990) Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 72:403–408
Boos N, Rieder R, Schade V, Spratt KF, Semmer N, Aebi M (1995) 1995 Volvo Award in clinical sciences. The diagnostic accuracy of magnetic resonance imaging, work perception, and psychosocial factors in identifying symptomatic disc herniation. Spine 20:2613–2625
Czéh B, Simon M, Schmelting B, Hiemke C, Fuchs E (2006) Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31:1616–1626
da Silva Torres IL, Cucco SN, Bassani M, Duarte MS, Silveira PP, Vasconcellos AP, Tabajara AS, Dantas G, Fontella FU, Dalmaz C, Ferreira MB (2003) Long-lasting delayed hyperalgesia after chronic restraint stress in rats—effect of morphine administration. Neurosci Res 45:277–283
Dietz DM, Dietz KC, Moore S, Ouimet CC, Kabbaj M (2008) Repeated social defeat stress-induced sensitization to the locomotor activating effects of d-amphetamine: role of individual differences. Psychopharmacology 198:51–62
Hasenbring M, Marienfield G, Kuhlendahl D, Soyka D (1994) Risk factor of chronicity in lumbar disc patients. Spine 24:2759–2765
Hatashita S, Sekiguchi M, Kobayashi H, Konno S, Kikuchi S (2008) Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine (Phila Pa 1976) 33:1344–1351
Hinwood M, Morandini J, Day TA, Walker FR (2012) Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex 22:1442–1454
Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M (2010) The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague–Dawley rats. Psychopharmacology 211:69–77
Igarashi T, Kikuchi S, Shubayev V, Myers RR (2000) 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine (Phila Pa 1976) 25:2975–2980
Imbe H, Iwai-Liao Y, Senba E (2006) Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci 11:2179–2192
Imbe H, Kimura A, Donishi T, Kaneoke Y (2012) Chronic restraint stress decreases glial fibrillary acidic protein and glutamate transporter in the periaqueductal gray matter. Neuroscience 223:209–218
Imbe H, Kimura A, Donishi T, Kaneoke Y (2013) Effects of restraint stress on glial activity in the rostral ventromedial medulla. Neuroscience 241:10–21
Joëls M, Krugers HJ (2007) LTP after stress: up or down? Neural Plast 2007:93202
Kameda T, Sekiguchi M, Kaneuchi Y, Konno S (2017) Investigation of the effect of diabetes on radiculopathy induced by nucleus pulposus application to the DRG in a spontaneously diabetic rat model. Spine (Phila Pa 1976) 42:1749–1756
Kaneko H, Zhang S, Sekiguchi M, Nikaido T, Kurata J, Konno S (2017) Dysfunction of nucleus accumbens is associated with psychiatric problems in patients with chronic low back pain: a functional magnetic resonance imaging study. Spine (Phila Pa 1976) 42:844–853
Kaneuchi Y, Sekiguchi M, Kameda T, Kobayashi Y, Konno S (2018) Temporal and spatial changes of μ-opioid receptors in the brain, spinal cord and dorsal root ganglion in a rat lumbar disc herniation model. Spine (Phila Pa 1976) 44:85–95
Kato K, Kikuchi S, Konno S, Sekiguchi M (2008) Participation of 5-hydroxytryptamine in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Spine (Phila Pa 1976) 33:1330–1336
Kawakami M, Tamaki T, Weinstein JN, Hashizume H, Nishi H, Meller ST (1996) Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine (Phila Pa 1976) 21:2101–2107
Kayama S, Konno S, Olmarker K, Yabuki S, Kikuchi S (1996) Incision of the anulus fibrosus induces nerve root morphologic, vascular, and functional changes. An experimental study. Spine (Phila Pa 1976) 21:2539–2543
Kim WJ, Kang H, Kim JE, Choi GJ, Shin HY, Baek CW, Jung YH, Woo YC, Kim SH, Lee JH (2014) Effect of intraperitoneal administered ginseng total saponins on hyperalgesia induced by repeated intramuscular injection of acidic saline in rats. J Med Food 17:657–662
Kobayashi H, Kikuchi S, Konno S, Kato K, Sekiguchi M (2011) Interaction of 5-hydroxytryptamine and tumor necrosis factor-α to pain-related behavior by nucleus pulposus applied on the nerve root in rats. Spine (Phila Pa 1976) 36:210–218
Kobayashi Y, Sekiguchi M, Konno S (2017) Effect of an acid-sensing ion channels inhibitor on pain-related behavior by nucleus pulposus applied on the nerve root in rats. Spine (Phila Pa 1976) 42:E633–E641
Konno S-I, Sekiguchi M (2018) Association between brain and low back pain. J Orthop Sci 23:3–7
Kovacs FM, Abraira V, Zamora J, Gil Teresa, del Real M, Llobera J, Fernández C, Bauza JR, Bauza K, Coll J, Cuadri M, Duro E, Gili J, Gestoso M, Gómez M, González J, Ibañez P, Jover A, Lázaro P, Llinás M, Mateu C, Mufraggi N, Muriel A, Nicolau C, Olivera MA, Pascual P, Perelló L, Pozo F, Revuelta T, Reyes V, Ribot S, Ripoll J, Ripoll J, Rodríguez E, Kovacs-Atención Primaria Group (2004) Correlation between pain, disability, and quality of life in patients with common low back pain. Spine (Phila Pa 1976) 29:206–210
McCarron RF, Wimpee MW, Hudkins PG, Laros GS (1987) The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine (Phila Pa 1976) 12:760–764
Miczek KA, Yap JJ, Covington HE (2008) Social stress, therapeutics and drug abuse: preclinicalmodels of escalated and depressed intake. Pharmacol Ther 120:102–128
Miyoshi S, Sekiguchi M, Konno S, Kikuchi S, Kanaya F (2011) Increased expression of vascular endothelial growth factor protein in dorsal root ganglion exposed to nucleus pulposus on the nerve root in rats. Spine 36:E1–E6
Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M (2006) Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain 7:816–822
O’Callaghan JP, Brinton RE, McEwen BS (1989) Glucocorticoids regulate the concentration of glial fibrillary acidic protein throughout the brain. Brain Res 494:159–161
Ohtori S, Takahashi K, Moriya H, Myers MM (2004) TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976) 29:1082–1088
Olmarker K, Rydevik B, Nordborg C (1993) Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine (Phila Pa 1976) 18:1425–1432
Olmarker K, Brisby H, Yabuki S, Nordborg C, Rydevik R (1997) The effects of normal, frozen, and hyaluronidase-digested nucleus pulposus on nerve root structure and function. Spine (Phila Pa 1976) 22:471–475 (discussion 476)
Olmarker K (2008) Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior. Spine 33:850–855
Otoshi K, Kikuchi S, Konno S, Sekiguchi M (2010) The reactions of glial cells and endoneurial macrophages in the dorsal root ganglion and their contribution to pain-related behavior after application of nucleus pulposus onto the nerve root in rats. Spine (Phila Pa 1976) 35:264–271
Paxinos G, Watson G (2007) The rat brain (in stereotaxic coordinates), vol 46, 6th edn. Academic Press, New York
Pincus T, Burton AK, Vogel S, Field AP (2002) A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976) 27:E109–E120
Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H (2000) Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav 67:449–458
Saito H, Wakai J, Sekiguchi M, Kikuchi S, Konno S (2014) The effect of selective serotonin reuptake inhibitor (SSRI) on pain-related behavior in a rat model of neuropathic pain. Eur Spine J 23:2401–2409
Satoh M, Kuraishi Y, Kawamura M (1992) Effects of intrathecal antibodies to substance P, calcitonin gene-related peptide and galanin on repeated cold stress-induced hyperalgesia: comparison with carrageenan-induced hyperalgesia. Pain 49:273–278
Seki S, Sekiguchi M, Konno S (2018) Association between neurotrophic factor expression and pain-related behavior induced by nucleus pulposus applied to rat nerve root. Spine (Phila Pa 1976) 43:E7–E15
Takahashi N, Kikuchi S, Konno S, Morita S, Suzukamo Y, Green J, Fukuhara S (2006) Discrepancy between disability and the severity of low back pain: demographic, psychologic, and employment-related factors. Spine (Phila Pa 1976) 31:931–939 (discussion 940)
Takeda M, Tanimoto T, Kadoi J, Masu M, Takahashi M, Kitagawa J, Matsumoto S (2007) Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain 129:155–166
Toth I, Neumann ID (2013) Animal models of social avoidance and social fear. Cell Tissue Res 354:107–118
Toyoda A (2017) Social defeat models in animal science: what we have learned from rodent models. Anim Sci J 88:944–952
Uesugi K, Sekiguchi M, Kikuchi S, Konno S (2011) The effect of repeated restraint stress in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Eur Spine J 20:1885–1891
Watkins LR, Maier SF (2002) Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 82:981–1011
Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF (2011) β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31:6277–6288
Ye Y, Wang G, Wang H, Wang X (2011) Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci Lett 503:15–19
Acknowledgements
The authors thank Akira Sato for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
IRB approval/Research Ethics Committee: There are no potential conflicts of interest in this study.
Ethical approval
The animal experiments were carried out under the supervision of the Animal Care and Use Committee in accordance with the Guidelines for Animal Experiments of our institution and the Government Law Concerning the Protection and Control of Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yomogida, S., Sekiguchi, M. & Konno, Si. Involvement between social defeat stress and pain-related behavior in a rat lumbar disk herniation model. Eur Spine J 29, 2431–2440 (2020). https://doi.org/10.1007/s00586-020-06533-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06533-1