Abstract
Purpose
To determine the relationship between the bone formation-related functions of GPR126 and the structural asymmetry of spine in adolescent idiopathic scoliosis (AIS).
Methods
Vertebral body samples were obtained from 51 AIS patients during spinal surgery between October 2014 and November 2017, and the expression pattern of GPR126 in the convex/concave sides of AIS spine was identified by RT-qPCR. Next, we explored the bone formation-related functions of GPR126 by knocking down and overexpressing GPR126 in human mesenchymal stem cells (hMSC) and further performing osteogenic differentiation. We also applied overexpression of N-terminal fragments derived from GPR126 (GPR126-NTFs) and osteogenic differentiation experiments to determine the functional part of GPR126 in skeletal development.
Results
We provided evidence that GPR126 showed a marked convex/concave asymmetric expression in the spine of AIS. Further RNA detection found that exon6-included transcripts of GPR126 (GPR126-exon6in) were significantly higher expressed in the convex side of AIS patients. Knocking down of GPR126 accelerated ossification of hMSCs during osteogenic differentiation, and overexpression of GPR126-exon6in delayed this process. Overexpression of GPR126-NTFs revealed that NTF is a functional fragment and exon6-included NTF (NTF-exon6in) delayed ossification of hMSCs.
Conclusion
Our findings indicated that GPR126-NTFs play a role in skeletal development, and the inclusion/exclusion of exon6 may regulate the bone formation-related functions of GPR126. The convex/concave asymmetric expression of GPR126-exon6in may be an important factor in abnormal bone formation of AIS.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.




Similar content being viewed by others
References
Xiong B, Sevastik B, Sevastik J, Hedlund R, Suliman I, Kristjansson S (1995) Horizontal plane morphometry of normal and scoliotic vertebrae. A methodological study. Eur Spine J 4(1):6–10
Xiong B, Sevastik B, Willers U, Sevastik J, Hedlund R (1995) Structural vertebral changes in the horizontal plane in idiopathic scoliosis and the long-term corrective effect of spine instrumentation. Eur Spine J 4(1):11–14
Liljenqvist UR, Allkemper T, Hackenberg L, Link TM, Steinbeck J, Halm HF (2002) Analysis of vertebral morphology in idiopathic scoliosis with use of magnetic resonance imaging and multiplanar reconstruction. J Bone Joint Surg Am 84-A(3):359–368
Adam CJ, Askin GN (2009) Lateral bone density variations in the scoliotic spine. Bone 45(4):799–807. https://doi.org/10.1016/j.bone.2009.06.023
Millner PA, Dickson RA (1996) Idiopathic scoliosis: biomechanics and biology. Eur Spine J 5(6):362–373
Yim AP, Yeung HY, Hung VW, Lee KM, Lam TP, Ng BK, Qiu Y, Cheng JC (2012) Abnormal skeletal growth patterns in adolescent idiopathic scoliosis—a longitudinal study until skeletal maturity. Spine (Phila Pa 1976) 37(18):E1148–E1154. https://doi.org/10.1097/brs.0b013e31825c036d
Lee WT, Cheung CS, Tse YK, Guo X, Qin L, Ho SC, Lau J, Cheng JC (2005) Generalized low bone mass of girls with adolescent idiopathic scoliosis is related to inadequate calcium intake and weight bearing physical activity in peripubertal period. Osteoporos Int J Establ Result Cooperation Betw Eur Found Osteoporos Natl Osteoporos Found USA 16(9):1024–1035. https://doi.org/10.1007/s00198-004-1792-1
Chiru M (2011) Adolescent idiopathic scoliosis and osteopenia). Maedica 6(1):17–22
Sadat-Ali M, Al-Othman A, Bubshait D, Al-Dakheel D (2008) Does scoliosis causes low bone mass? A comparative study between siblings. Eur Spine J 17(7):944–947. https://doi.org/10.1007/s00586-008-0671-4
Hung VW, Qin L, Cheung CS, Lam TP, Ng BK, Tse YK, Guo X, Lee KM, Cheng JC (2005) Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am 87(12):2709–2716. https://doi.org/10.2106/JBJS.D.02782
Yu WS, Chan KY, Yu FW, Ng BK, Lee KM, Qin L, Lam TP, Cheng JC (2014) Bone structural and mechanical indices in Adolescent Idiopathic Scoliosis evaluated by high-resolution peripheral quantitative computed tomography (HR-pQCT). Bone 61:109–115. https://doi.org/10.1016/j.bone.2013.12.033
Wang Z, Chen H, Yu YE, Zhang J, Cheuk KY, Ng BK, Qiu Y, Guo XE, Cheng JC, Lee WY (2017) Unique local bone tissue characteristics in iliac crest bone biopsy from adolescent idiopathic scoliosis with severe spinal deformity. Sci Rep 7:40265. https://doi.org/10.1038/srep40265
Tam EM, Yu FW, Hung VW, Liu Z, Liu KL, Ng BK, Lee SK, Qiu Y, Cheng JC, Lam TP (2014) Are volumetric bone mineral density and bone micro-architecture associated with leptin and soluble leptin receptor levels in adolescent idiopathic scoliosis?—A case-control study. PLoS ONE 9(2):e87939. https://doi.org/10.1371/journal.pone.0087939
Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, Qiu X, Sharma S, Takimoto A, Ogura Y, Jiang H, Yan H, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Hosono N, Tsuji T, Suzuki T, Sudo H, Kotani T, Yonezawa I, Londono D, Gordon D, Herring JA, Watanabe K, Chiba K, Kamatani N, Jiang Q, Hiraki Y, Kubo M, Toyama Y, Tsunoda T, Wise CA, Qiu Y, Shukunami C, Matsumoto M, Ikegawa S (2013) Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet 45(6):676–679
Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP (2015) Association of GPR126 gene polymorphism with adolescent idiopathic scoliosis in Chinese populations. Genomics 105(2):101–107
Qin X, Xu L, Xia C, Zhu W, Sun W, Liu Z, Qiu Y, Zhu Z (2017) Genetic variant of GPR126 gene is functionally associated with adolescent idiopathic scoliosis in Chinese population. Spine (Phila Pa 1976) 42(19):E1098–E1103. https://doi.org/10.1097/brs.0000000000002123
Liu G, Liu S, Lin M, Li X, Chen W, Zuo Y, Liu J, Niu Y, Zhao S, Long B, Wu Z, Wu N, Qiu G (2018) Genetic polymorphisms of GPR126 are functionally associated with PUMC classifications of adolescent idiopathic scoliosis in a Northern Han population. J Cell Mol Med 22:1964–1971
Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, Eyheramendy S, Voight BF, Butler JL, Guiducci C, Illig T, Hackett R, Heid IM, Jacobs KB, Lyssenko V, Uda M, Boehnke M, Chanock SJ, Groop LC, Hu FB, Isomaa B, Kraft P, Peltonen L, Salomaa V, Schlessinger D, Hunter DJ, Hayes RB, Abecasis GR, Wichmann HE, Mohlke KL, Hirschhorn JN (2008) Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet 40(5):584–591
Soranzo N, Rivadeneira F, Chinappen-Horsley U, Malkina I, Richards JB, Hammond N, Stolk L, Nica A, Inouye M, Hofman A, Stephens J, Wheeler E, Arp P, Gwilliam R, Jhamai PM, Potter S, Chaney A, Ghori MJ, Ravindrarajah R, Ermakov S, Estrada K, Pols HA, Williams FM, McArdle WL, van Meurs JB, Loos RJ, Dermitzakis ET, Ahmadi KR, Hart DJ, Ouwehand WH, Wareham NJ, Barroso I, Sandhu MS, Strachan DP, Livshits G, Spector TD, Uitterlinden AG, Deloukas P (2009) Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet 5(4):e1000445
Sovio U, Bennett AJ, Millwood IY, Molitor J, O’Reilly PF, Timpson NJ, Kaakinen M, Laitinen J, Haukka J, Pillas D, Tzoulaki I, Molitor J, Hoggart C, Coin LJ, Whittaker J, Pouta A, Hartikainen AL, Freimer NB, Widen E, Peltonen L, Elliott P, McCarthy MI, Jarvelin MR (2009) Genetic determinants of height growth assessed longitudinally from infancy to adulthood in the northern Finland birth cohort 1966. PLoS Genet 5(3):e1000409
Zhao J, Li M, Bradfield JP, Zhang H, Mentch FD, Wang K, Sleiman PM, Kim CE, Glessner JT, Hou C, Keating BJ, Thomas KA, Garris ML, Deliard S, Frackelton EC, Otieno FG, Chiavacci RM, Berkowitz RI, Hakonarson H, Grant SF (2010) The role of height-associated loci identified in genome wide association studies in the determination of pediatric stature. BMC Med Genet 11:96
Monk KR, Oshima K, Jors S, Heller S, Talbot WS (2011) Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 138(13):2673–2680
Moriguchi T, Haraguchi K, Ueda N, Okada M, Furuya T, Akiyama T (2004) DREG, a developmentally regulated G protein-coupled receptor containing two conserved proteolytic cleavage sites. Genes Cells 9(6):549–560. https://doi.org/10.1111/j.1356-9597.2004.00743.x
Langenhan T, Aust G, Hamann J (2013) Sticky signaling–adhesion class G protein-coupled receptors take the stage. Sci Signal 6(276):re3. https://doi.org/10.1126/scisignal.2003825
Yip BH, Yu FW, Wang Z, Hung VW, Lam TP, Ng BK, Zhu F, Cheng JC (2016) Prognostic value of bone mineral density on curve progression: a longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep 6:39220. https://doi.org/10.1038/srep39220
Tanabe H, Aota Y, Yamaguchi Y, Kaneko K, Imai S, Takahashi M, Taguri M, Saito T (2018) Minodronate treatment improves low bone mass and reduces progressive thoracic scoliosis in a mouse model of adolescent idiopathic scoliosis. PLoS ONE 13(8):e0202165. https://doi.org/10.1371/journal.pone.0202165
Suh KT, Lee SS, Hwang SH, Kim SJ, Lee JS (2007) Elevated soluble receptor activator of nuclear factor-κB ligand and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J 16(10):1563–1569. https://doi.org/10.1007/s00586-007-0390-2
Man GC, Wong JH, Wang WW, Sun GQ, Yeung BH, Ng TB, Lee SK, Ng BK, Qiu Y, Cheng JC (2011) Abnormal melatonin receptor 1B expression in osteoblasts from girls with adolescent idiopathic scoliosis. J Pineal Res 50(4):395–402. https://doi.org/10.1111/j.1600-079X.2011.00857.x
Fendri K, Patten SA, Kaufman GN, Zaouter C, Parent S, Grimard G, Edery P, Moldovan F (2013) Microarray expression profiling identifies genes with altered expression in adolescent idiopathic scoliosis. Eur Spine J 22(6):1300–1311. https://doi.org/10.1007/s00586-013-2728-2
Zhuang Q, Li J, Wu Z, Zhang J, Sun W, Li T, Yan Y, Jiang Y, Zhao RC, Qiu G (2011) Differential proteome analysis of bone marrow mesenchymal stem cells from adolescent idiopathic scoliosis patients. PLoS ONE 6(4):e18834. https://doi.org/10.1371/journal.pone.0018834
Zhou T, Chen C, Xu C, Zhou H, Gao B, Su D, Liao Z, Li Y, Yang S, Su P (2018) Mutant MAPK7-induced idiopathic scoliosis is linked to impaired osteogenesis. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 48(3):880–890. https://doi.org/10.1159/000491956
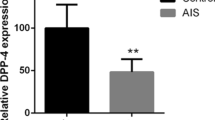
Normand E, Franco A, Moreau A, Marcil V (2017) Dipeptidyl peptidase-4 and adolescent idiopathic scoliosis: expression in osteoblasts. Sci Rep 7(1):3173. https://doi.org/10.1038/s41598-017-03310-x
Man GC, Wang WW, Yeung BH, Lee SK, Ng BK, Hung WY, Wong JH, Ng TB, Qiu Y, Cheng JC (2010) Abnormal proliferation and differentiation of osteoblasts from girls with adolescent idiopathic scoliosis to melatonin. J Pineal Res 49(1):69–77. https://doi.org/10.1111/j.1600-079X.2010.00768.x
Zhuang Q, Ye B, Hui S, Du Y, Zhao RC, Li J, Wu Z, Li N, Zhang Y, Li H, Wang S, Yang Y, Li S, Zhao H, Fan Z, Qiu G, Zhang J (2018) Long noncoding RNA lncAIS downregulation in mesenchymal stem cells is implicated in the pathogenesis of adolescent idiopathic scoliosis. Cell Death Differ. https://doi.org/10.1038/s41418-018-0240-2
Zhang J, Chen H, Leung RKK, Choy KW, Lam TP, Ng BKW, Qiu Y, Feng JQ, Cheng JCY, Lee WYW (2018) Aberrant miR-145-5p/β-catenin signal impairs osteocyte function in adolescent idiopathic scoliosis. FASEB J 45:45. https://doi.org/10.1096/fj.201800281
Garcia-Gimenez JL, Rubio-Belmar PA, Peiro-Chova L, Hervas D, Gonzalez-Rodriguez D, Ibanez-Cabellos JS, Bas-Hermida P, Mena-Molla S, Garcia-Lopez EM, Pallardo FV, Bas T (2018) Circulating miRNAs as diagnostic biomarkers for adolescent idiopathic scoliosis. Sci Rep 8(1):2646. https://doi.org/10.1038/s41598-018-21146-x
Oliazadeh N, Gorman KF, Eveleigh R, Bourque G, Moreau A (2017) Identification of elongated primary cilia with impaired mechanotransduction in idiopathic scoliosis patients. Sci Rep 7:44260. https://doi.org/10.1038/srep44260
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42(4):606–615. https://doi.org/10.1016/j.bone.2007.12.224
Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, Rubin CT (2010) Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol 6(1):50–59. https://doi.org/10.1038/nrrheum.2009.239
Harada S, Rodan GA (2003) Control of osteoblast function and regulation of bone mass. Nature 423(6937):349–355. https://doi.org/10.1038/nature01660
Pollak L, Shlamkovic N, Minewicz A, Mirovsky Y (2013) Otolith dysfunction as a possible cause for the development of idiopathic scoliosis. J Pediatr Orthop 33(3):293–297
Kitagaki J, Miyauchi S, Asano Y, Imai A, Kawai S, Michikami I, Yamashita M, Yamada S, Kitamura M, Murakami S (2016) A putative association of a single nucleotide polymorphism in GPR126 with aggressive periodontitis in a Japanese population. PLoS ONE 11(8):e0160765. https://doi.org/10.1371/journal.pone.0160765
Lorson CL, Hahnen E, Androphy EJ, Wirth B (1999) A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA 96(11):6307–6311
Albert H, Santos S, Battaglia E, Brito M, Monteiro C, Bagrel D (2011) Differential expression of CDC25 phosphatases splice variants in human breast cancer cells. Clin Chem Lab Med 49(10):1707–1714. https://doi.org/10.1515/CCLM.2011.635
Wang Y, Chan DW, Liu VW, Chiu P, Ngan HY (2010) Differential functions of growth factor receptor-bound protein 7 (GRB7) and its variant GRB7v in ovarian carcinogenesis. Clin Cancer Res 16(9):2529–2539. https://doi.org/10.1158/1078-0432.CCR-10-0018
Han GS, Carman GM (2010) Characterization of the human LPIN1-encoded phosphatidate phosphatase isoforms. J Biol Chem 285(19):14628–14638. https://doi.org/10.1074/jbc.M110.117747
Zhang P, Takeuchi K, Csaki LS, Reue K (2012) Lipin-1 phosphatidic phosphatase activity modulates phosphatidate levels to promote peroxisome proliferator-activated receptor gamma (PPARgamma) gene expression during adipogenesis. J Biol Chem 287(5):3485–3494. https://doi.org/10.1074/jbc.M111.296681
Khankin EV, Mutter WP, Tamez H, Yuan HT, Karumanchi SA, Thadhani R (2010) Soluble erythropoietin receptor contributes to erythropoietin resistance in end-stage renal disease. PLoS ONE 5(2):e9246. https://doi.org/10.1371/journal.pone.0009246
Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30(6):586–623. https://doi.org/10.1210/er.2008-0047
Fredriksson R, Gloriam DE, Hoglund PJ, Lagerstrom MC, Schioth HB (2003) There exist at least 30 human G-protein-coupled receptors with long Ser/Thr-rich N-termini. Biochem Biophys Res Commun 301(3):725–734
Stehlik C, Kroismayr R, Dorfleutner A, Binder BR, Lipp J (2004) VIGR—a novel inducible adhesion family G-protein coupled receptor in endothelial cells. FEBS Lett 569(1–3):149–155. https://doi.org/10.1016/j.febslet.2004.05.038
Patra C, van Amerongen MJ, Ghosh S, Ricciardi F, Sajjad A, Novoyatleva T, Mogha A, Monk KR, Muhlfeld C, Engel FB (2013) Organ-specific function of adhesion G protein-coupled receptor GPR126 is domain-dependent. Proc Natl Acad Sci USA 110(42):16898–16903. https://doi.org/10.1073/pnas.1304837110
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, E., Lin, T., Jiang, H. et al. Asymmetric expression of GPR126 in the convex/concave side of the spine is associated with spinal skeletal malformation in adolescent idiopathic scoliosis population. Eur Spine J 28, 1977–1986 (2019). https://doi.org/10.1007/s00586-019-06001-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-06001-5