Abstract
Purpose
Intervertebral disk (IVD) lesion and its subsequent degeneration have a profound effect on the multifidus muscle. The subacute/early chronic phase of multifidus remodeling after IVD lesion has been proposed to be regulated by inflammatory processes. The balance between pro-inflammatory (M1) and anti-inflammatory (M2) macrophages plays an important role in maintaining tissue integrity after injury. The localization, polarization of macrophage subtypes and their mediation of the pro-inflammatory cytokine tumor necrosis factor (TNF) are unknown in paraspinal muscles during IVD degeneration. A sheep model of IVD degeneration was used to investigate the role of macrophages and TNF in the structural alterations that occur within the multifidus muscle.
Methods
Anterolateral lesions were induced at L3–4 IVD in sheep. Multifidus muscle tissue at L4 was harvested 3 and 6 months after lesion and used for immunofluorescence assays to examine total macrophage number, macrophage polarization between M1 and M2, and to assess the localization of TNF expression in muscle, adipose and connective tissues from injured and naïve control animals.
Results
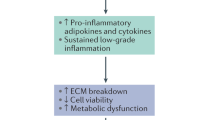
A greater proportion of M1 macrophages is present in muscle at both 3 and 6 months after IVD lesion, and adipose tissue at 6 months. Total number of macrophages is unchanged. At 6 months, expression of TNF is increased in adipose and connective tissue and the proportion of TNF expressed by M1 macrophages is increased.
Conclusions
These data support the proposal that macrophages and TNF (pro-inflammatory cytokine) play an active role in the subacute/early chronic phase of remodeling in muscle, adipose and connective tissues of the multifidus during IVD degeneration. This presents a novel target for treatment.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.









Similar content being viewed by others
References
Vos T, Flaxman AD, Naghavi M et al (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 380(9859):2163–2196. https://doi.org/10.1016/s0140-6736(12)61729-2
Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, Williams G, Smith E, Vos T, Barendregt J, Murray C, Burstein R, Buchbinder R (2014) The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 73(6):968–974. https://doi.org/10.1136/annrheumdis-2013-204428
Phillips CJ (2009) The cost and burden of chronic pain. Rev Pain 3(1):2–5. https://doi.org/10.1177/204946370900300102
Li Y, Liu J, Liu ZZ, Duan DP (2016) Inflammation in low back pain may be detected from the peripheral blood: suggestions for biomarker. Biosci Rep. https://doi.org/10.1042/bsr20160187
Weber KT, Alipui DO, Sison CP, Bloom O, Quraishi S, Overby MC, Levine M, Chahine NO (2016) Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Therapy 18:3. https://doi.org/10.1186/s13075-015-0887-8
Leung A, Gregory NS, Allen LA, Sluka KA (2016) Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain 157(1):70–79. https://doi.org/10.1097/j.pain.0000000000000312
Hodges PW, James G, Blomster L, Hall L, Schmid AB, Shu C, Little C, Melrose J (2014) Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine 39(13):1010–1017. https://doi.org/10.1097/brs.0000000000000318
Hodges PW, James G, Blomster L, Hall L, Schmid A, Shu C, Little C, Melrose J (2015) Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine 40(14):1057–1071. https://doi.org/10.1097/brs.0000000000000972
James G, Blomster L, Hall L, Schmid AB, Shu CC, Little CB, Melrose J, Hodges PW (2016) Mesenchymal stem cell treatment of intervertebral disc lesion prevents fatty infiltration and fibrosis of the multifidus muscle, but not cytokine and muscle fiber changes. Spine 41(15):1208–1217. https://doi.org/10.1097/brs.0000000000001669
Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N (1995) The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine 20(17):1878–1883
Molinos M, Almeida CR, Caldeira J, Cunha C, Goncalves RM, Barbosa MA (2015) Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface R Soc 12(104):20141191. https://doi.org/10.1098/rsif.2014.1191
Hodges P, Holm AK, Hansson T, Holm S (2006) Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine 31(25):2926–2933. https://doi.org/10.1097/01.brs.0000248453.51165.0b
Hodges PW, Galea MP, Holm S, Holm AK (2009) Corticomotor excitability of back muscles is affected by intervertebral disc lesion in pigs. Eur J Nurosci 29(7):1490–1500. https://doi.org/10.1111/j.1460-9568.2009.06670.x
Brown SH, Gregory DE, Carr JA, Ward SR, Masuda K, Lieber RL (2011) ISSLS prize winner: adaptations to the multifidus muscle in response to experimentally induced intervertebral disc degeneration. Spine 36(21):1728–1736. https://doi.org/10.1097/BRS.0b013e318212b44b
Zhao WP, Kawaguchi Y, Matsui H, Kanamori M, Kimura T (2000) Histochemistry and morphology of the multifidus muscle in lumbar disc herniation: comparative study between diseased and normal sides. Spine 25(17):2191–2199
Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH (1994) Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine 19(2):165–172
Alaranta H, Tallroth K, Soukka A, Heliovaara M (1993) Fat content of lumbar extensor muscles and low back disability: a radiographic and clinical comparison. J Spinal Disord 6(2):137–140
Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C (2007) Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med 5:2. https://doi.org/10.1186/1741-7015-5-2
Fortin M, Lazary A, Varga PP, McCall I, Battie MC (2016) Paraspinal muscle asymmetry and fat infiltration in patients with symptomatic disc herniation. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 25(5):1452–1459. https://doi.org/10.1007/s00586-016-4503-7
Sun D, Liu P, Cheng J, Ma Z, Liu J, Qin T (2017) Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. BMC Musculoskelet Disord 18(1):167. https://doi.org/10.1186/s12891-017-1522-4
Schafers M, Sorkin LS, Sommer C (2003) Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain 104(3):579–588
Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA (2016) Resident macrophages in muscle contribute to development of hyperalgesia in a mouse model of noninflammatory muscle pain. J Pain Off J Am Pain Soc. https://doi.org/10.1016/j.jpain.2016.06.010
Gregory NS, Brito RG, Fusaro MC, Sluka KA (2016) ASIC3 is required for development of fatigue-induced hyperalgesia. Mol Neurobiol 53(2):1020–1030. https://doi.org/10.1007/s12035-014-9055-4
Wehling-Henricks M, Jordan MC, Gotoh T, Grody WW, Roos KP, Tidball JG (2010) Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS ONE 5(5):e10763. https://doi.org/10.1371/journal.pone.0010763
Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG (2009) Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet 18(3):482–496. https://doi.org/10.1093/hmg/ddn376
Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P (2011) Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1(1):21. https://doi.org/10.1186/2044-5040-1-21
da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR (2015) IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol 51(1):19–31. https://doi.org/10.1007/s12035-014-8790-x
Shu CC, Dart A, Clarke EC, Martin J, Little CB, Melrose J (2014) Prevention and treatment of intervertebral disc degeneration with bone marrow derived stem (stromal) cells; an in vivo study in sheep. Osteoarthr Cartil 22:S28–S29. https://doi.org/10.1016/j.joca.2014.02.074
Shu CC, Smith MM, Smith SM, Dart AJ, Little CB, Melrose J (2017) A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells for intervertebral disc regeneration. Int J Mol Sci. https://doi.org/10.3390/ijms18051049
Melrose J, Shu C, Young C, Ho R, Smith MM, Young AA, Smith SS, Gooden B, Dart A, Podadera J, Appleyard RC, Little CB (2012) Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine 37(1):18–25. https://doi.org/10.1097/BRS.0b013e31820cd8d5
McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A (2014) Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell cycle (Georgetown, Tex) 13(9):1400–1412. https://doi.org/10.4161/cc.28401
Wang N, Liang H, Zen K (2014) Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol 5:614. https://doi.org/10.3389/fimmu.2014.00614
Yokoyama T, Lisi TL, Moore SA, Sluka KA (2007) Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain Off J Am Pain Soc 8(9):692–699. https://doi.org/10.1016/j.jpain.2007.05.008
Gregory NS, Whitley PE, Sluka KA (2015) Effect of intramuscular protons, lactate, and ATP on muscle hyperalgesia in rats. PLoS ONE 10(9):e0138576. https://doi.org/10.1371/journal.pone.0138576
Roy SH, De Luca CJ, Snyder-Mackler L, Emley MS, Crenshaw RL, Lyons JP (1990) Fatigue, recovery, and low back pain in varsity rowers. Med Sci Sports Exerc 22(4):463–469
Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T (2008) Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain 140(2):254–264. https://doi.org/10.1016/j.pain.2008.08.014
Steen KH, Issberner U, Reeh PW (1995) Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neurosci Lett 199(1):29–32
Novak ML, Weinheimer-Haus EM, Koh TJ (2014) Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol 232(3):344–355. https://doi.org/10.1002/path.4301
Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204(5):1057–1069. https://doi.org/10.1084/jem.20070075
St Pierre BA, Tidball JG (1994) Differential response of macrophage subpopulations to soleus muscle reloading after rat hind limb suspension. J Appl Physiol (Bethesda, Md : 1985) 77(1):290–297
Munoz-Canoves P, Serrano AL (2015) Macrophages decide between regeneration and fibrosis in muscle. Trends Endocrinol Metab TEM 26(9):449–450. https://doi.org/10.1016/j.tem.2015.07.005
Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM (2015) Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21(7):786–794. https://doi.org/10.1038/nm.3869
Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, Tidball JG (2011) Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet 20(4):790–805. https://doi.org/10.1093/hmg/ddq523
Kuru S, Inukai A, Kato T, Liang Y, Kimura S, Sobue G (2003) Expression of tumor necrosis factor-alpha in regenerating muscle fibers in inflammatory and non-inflammatory myopathies. Acta Neuropathol 105(3):217–224. https://doi.org/10.1007/s00401-002-0635-4
Meshkani R, Vakili S (2016) Tissue resident macrophages: key players in the pathogenesis of type 2 diabetes and its complications. Clin Chim Acta Int J Clin Chem 462:77–89. https://doi.org/10.1016/j.cca.2016.08.015
Gumucio JP, Davis ME, Bradley JR, Stafford PL, Schiffman CJ, Lynch EB, Claflin DR, Bedi A, Mendias CL (2012) Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res Off Publ Orthop Res Soc 30(12):1963–1970. https://doi.org/10.1002/jor.22168
Gumucio JP, Korn MA, Saripalli AL, Flood MD, Phan AC, Roche SM, Lynch EB, Claflin DR, Bedi A, Mendias CL (2014) Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg Am Shoulder Elbow Surg [et al] 23(1):99–108. https://doi.org/10.1016/j.jse.2013.04.011
Serrano-Marco L, Chacon MR, Maymo-Masip E, Barroso E, Salvado L, Wabitsch M, Garrido-Sanchez L, Tinahones FJ, Palomer X, Vendrell J, Vazquez-Carrera M (2012) TNF-alpha inhibits PPARbeta/delta activity and SIRT1 expression through NF-kappaB in human adipocytes. Biochem Biophys Acta 1821(9):1177–1185. https://doi.org/10.1016/j.bbalip.2012.05.006
Barish GD, Narkar VA, Evans RM (2006) PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Investig 116(3):590–597. https://doi.org/10.1172/jci27955
Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM (2004) Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2(10):e294. https://doi.org/10.1371/journal.pbio.0020294
Foresto CS, Paula-Gomes S, Silveira WA, Graca FA, KettelhutIdo C, Goncalves DA, Mattiello-Sverzut AC (2016) Morphological and molecular aspects of immobilization-induced muscle atrophy in rats at different stages of postnatal development: the role of autophagy. J Appl Physiol (Bethesda, Md: 1985) 121(3):646–660. https://doi.org/10.1152/japplphysiol.00687.2015
Yamada E, Bastie CC, Koga H, Wang Y, Cuervo AM, Pessin JE (2012) Mouse skeletal muscle fiber-type-specific macroautophagy and muscle wasting are regulated by a Fyn/STAT3/Vps34 signaling pathway. Cell Rep 1(5):557–569. https://doi.org/10.1016/j.celrep.2012.03.014
Keller CW, Fokken C, Turville SG, Lunemann A, Schmidt J, Munz C, Lunemann JD (2011) TNF-alpha induces macroautophagy and regulates MHC class II expression in human skeletal muscle cells. J Biol Chem 286(5):3970–3980. https://doi.org/10.1074/jbc.M110.159392
Wermuth PJ, Jimenez SA (2015) The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin Transl Med 4:2. https://doi.org/10.1186/s40169-015-0047-4
Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L (2009) Imatinib attenuates skeletal muscle dystrophy in MDX mice. FASEB J Off Publ Fed Am Soc Exp Biol 23(8):2539–2548. https://doi.org/10.1096/fj.09-129833
Ito T, Ogawa R, Uezumi A, Ohtani T, Watanabe Y, Tsujikawa K, Miyagoe-Suzuki Y, Takeda S, Yamamoto H, Fukada S (2013) Imatinib attenuates severe mouse dystrophy and inhibits proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord NMD 23(4):349–356. https://doi.org/10.1016/j.nmd.2012.10.025
Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC (2012) Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol 228(1):77–87. https://doi.org/10.1002/path.4054
Sainoh T, Orita S, Miyagi M, Inoue G, Kamoda H, Ishikawa T, Yamauchi K, Suzuki M, Sakuma Y, Kubota G, Oikawa Y, Inage K, Sato J, Nakata Y, Nakamura J, Aoki Y, Toyone T, Takahashi K, Ohtori S (2016) Single intradiscal administration of the tumor necrosis factor-alpha inhibitor, etanercept, for patients with discogenic low back pain. Pain Med 17(1):40–45. https://doi.org/10.1111/pme.12892
Andronis L, Kinghorn P, Qiao S, Whitehurst DG, Durrell S, McLeod H (2016) Cost-effectiveness of non-invasive and non-pharmacological interventions for low back pain: a systematic literature review. Appl Health Econ Health Policy. https://doi.org/10.1007/s40258-016-0268-8
Belavy DL, Albracht K, Bruggemann GP, Vergroesen PP, van Dieen JH (2016) Can exercise positively influence the intervertebral disc? Sports Med 46(4):473–485. https://doi.org/10.1007/s40279-015-0444-2
Goldspink G, Scutt A, Loughna PT, Wells DJ, Jaenicke T, Gerlach GF (1992) Gene expression in skeletal muscle in response to stretch and force generation. Am J Physiol 262(3 Pt 2):R356–R363
Funding
PWH has a Senior Principal Research Fellowship from the National Health and Medical Research Council (NHMRC) of Australia (APP1002190). The study was funded by a NHMRC Program Grant (ID631717) and Project Grant (APP1004032). The funding source did not play a role in investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Outside of this study, Kathleen A. Sluka recieved research funding from Medtronic, Inc, consultancy fees from Bayer Inc. and a book honorarium from IASP Press. The other authors declare they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
James, G., Sluka, K.A., Blomster, L. et al. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur Spine J 27, 1744–1756 (2018). https://doi.org/10.1007/s00586-018-5652-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5652-7