Abstract
Purpose
Spine surgery is usually associated with large amount of blood loss and blood transfusion. Excessive blood loss may cause hypotension, inadequate oxygenation of organs, necessitate allogeneic blood transfusion, and spinal epidural hematoma formation. Aprotinin, TXA, and EACA are antifibrinolytics currently offered as prophylactic agents to reduce surgery-associated blood loss. The purpose of this study was to assess the efficacy of using antifibrinolytic agents in reducing blood loss and blood transfusions in spine surgery.
Methods
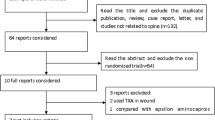
PubMed, Embase, and Cochrane-controlled trials register were used to identify RCTs published before April 2015 that examined the effectiveness of intravenous aprotinin, tranexamic acid (TXA), and epsilon-aminocaproic acid (EACA) on reduction of blood loss and blood transfusions, compared with placebo in spine surgery. Randomized controlled trials reported the primary outcome that included total blood loss, intra-operative blood loss, post-operative blood loss, blood transfusion requirements, blood transfusion rate, and incidence of deep vein thrombosis. Meta-analysis was performed using the Stata12.0. Weighted mean difference with 95 % confidence intervals was used to summarize the findings across the trials for continuous outcomes. Dichotomous data were expressed as risk ratios with 95 % confidence intervals. A P < 0.05 was considered statistically significant.
Results
17 studies involving 1191 patients were identified. Among them, 13 RCTs with 943 patients were included for the evaluation of total blood loss. Compared with the control group, the antifibrinolytic agents reduced total blood loss (SMD = −0.62; 95 % CI −0.75, −0.48; P = 0.000), The aprotinin group (SMD = −0.80; 95 % CI −1.22, −0.37; P = 0.938), The TXA group (SMD = −0.75; 95 % CI −0.93, −0.57; P = 0.000), and the EACA group (SMD = −0.28; 95 % CI −0.54, −0.01; P = 0.185). Thirteen RCTs with eight hundred and ninety four patients were included for the evaluation of intra-operative blood loss. Compared with the control group, the antifibrinolytic agents reduced intra-operative blood loss (SMD = −0.41; 95 % CI −0.55, −0.28; P = 0.010), The aprotinin group (SMD = −0.62; 95 % CI −0.93, −0.30; P = 0.862), The TXA group (SMD = −0.47; 95 % CI −0.64, −0.29; P = 0.005), and the EACA group (SMD = −0.16; 95 % CI −0.42, −0.11; P = 0.897). Eight RCTs with six hundred and seven patients were included for the evaluation of post-operative blood loss. Compared with the control group, the antifibrinolytic agents reduced post-operative blood loss (SMD = −0.68; 95 % CI −0.85, −0.51; P = 0.000), the aprotinin group (SMD = −0.48; 95 % CI −0.85, −0.12; P = 0.036), the TXA group (SMD = −0.80; 95 % CI −1.01, −0.59; P = 0.000), and the EACA group (SMD = −0.32; 95 % CI −0.68, −0.04; P = 0.009). Ten RCTs with seven hundred and twenty twopatients were included for the evaluation of blood transfusion. Compared with the control group, the antifibrinolytic agents reduced blood transfusion (SMD = −0.68; 95 % CI −0.85, −0.51; P = 0.000), the aprotinin group (SMD = −0.80; 95 % CI −1.22, −0.37; P = 0.938), the TXA group (SMD = −0.38; 95 % CI −0.58, −0.18; P = 0.000), and the EACA group (SMD = −0.28; 95 % CI −0.54, −0.01; P = 0.185). Twelve RCTs with eight hundred and fifteenpatients were included for the evaluation of blood transfusion rate. The transfusion rate was 35.6 % in the patients with antifibrinolytic agents and 55.2 % in the patients with placebo (RR = 0.75; 95 % CI 0.63, 0.89; P = 0.939). All studies were included for the evaluation of safety, with a total of eight thromboembolic events reported overall (two in the experimental group and six in the control group).
Conclusions
The antifibrinolytic agents were able to reduce perioperative blood loss and transfusion requirements in spine surgery. TXA appeared more effective than aprotinin and EACA in reducing total blood loss, intra-operative blood loss, and blood transfusion according to the results of this analysis. The three groups in reducing the post-operative blood loss are significantly better than control groups. There was no evidence that the use of antifibrinolytic agents was a risk factor for thromboembolism in spine surgery. Further multicenter, large-sample, double-blind RCTs are required to confirm the efficacy and safety of the three antifibrinolytic agents in spine surgery.













Similar content being viewed by others
References
Verma K, Errico TJ, Vaz KM, Lonner BS (2010) A prospective, randomized, double-blinded single-site controlled study comparing blood loss prevention of tranexamic acid (TXA) to epsilon aminocaproic avid (EACA) for corrective spinal surgery. BMC Surg 10:13. doi:10.1186/1471-2482-10-13
Hassan N, Halanski M, Wincek J, Reischman D, Sanfilippo D et al (2011) Blood management in pediatric spinal deformity surgery: review of a 2-year experience. Transfusion 51:2133–2141. doi:10.1111/j.1537-2995,2011.03175.x
Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM (2001) A random trial of tranexamic acid to reduce blood transfusion for scoliosis. Anesth_Analg 93:82–87
Hu SS (2004) Blood loss in adult spinal surgery. Eur Spine J 13(Suppl 1):S3–S5
Mannucci PM, Levi M (2007) Prevention and treatment of major blood loss. N Engl J Med 3(56):2301–2311
Yonenobu K, Hosono N, Iwasaki M et al (1991) Neurologic complications of surgery for cervical compression myelopathy. Spine 16:1277–1282
Kebaish KM, Awad JN (2004) Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus 16:e1
Sokolowski MJ, Garvey TA, Perl J II et al (2008) Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine 33:108–113
Henry DA, Carless PA, Moxey AJ et al (2011) Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 1:CD001886
Yang B, Li H, Wang D et al (2013) Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spine surgery. PLoS One 8:e55436
Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO (2010) Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine (Phila Pa 1976) 35:235–239
Mannucci PM (1998) Hemostatic drugs. N Engl J Med 339:245–253
Schouten ES, van de Pol AC, Schouten AN, Turner NM, Jansen NJ, Bollen CW (2009) The effect of aprotinin, tranexamic acid, and aminocaproic acid on blood loss and use of blood products in major pediatric surgery: a meta-analysis. Pediatric critical care medicine 10(2):182–190. doi:10.1097/PCC.0b013e3181956d61
Brown JR, Birkmeyer NJ, O’Connor GT (2007) Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation 115(22):2801–2813. doi:10.1161/CIRCULATIONAHA.106.671222
Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM (2014) A systematic review and metaanalysis of the topical administration of tranexamic acid in total hip and knee replacement. The bone & joint journal. 96-B(8):1005–15. doi: 10.1302/0301-620X.96B8.33745
Higgins JP, Altman DG (2008) Assessing risk of bias in included studies, vol 2008. Wiley, pp 187–241. doi:10.1002/9780470712184.ch8
Lentschener C, Cottin P, Bouaziz H, Mercier FJ, Wolf M, Aljabi Y et al (1999) Reduction of blood loss and transfusion requirement by aprotinin in posterior lumbar spine fusion. Anesth Analg 89(3):590–597
Karapurkar AKA, Naik L (2002) Aprotinin, to reduced perioperative blood loss in scoliosis surgery Indian J Anaesth 46(5):378–80
Cole JW, Murray DJ, Snider RJ, Bassett GS, Bridwell KH, Lenke LG (2003) Aprotinin reduces blood loss during spinal surgery in children. Spine 28(21):2482–2485. doi:10.1097/01.BRS.0000090835.45437.7F
Khoshhal K, Mukhtar I, Clark P, Jarvis J, Letts M, Splinter W (2003) Efficacy of aprotinin in reducing blood loss in spinal fusion for idiopathic scoliosis. J Pediatr Orthop 23(5):661–664
Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F (2005) Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology 102(4):727–732
Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A et al. (2008) Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976) 33:2577–2580
Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H et al (2008) Tranexamic Acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg 107:1479–1486
Farrokhi MR, Kazemi AP, Eftekharian HR, Akbari K (2011) Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a randomized clinical trial. J Neurosurg Anesthesiol 23:290–296
Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I et al. (2011) Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila Pa 1976) 36:1913–1918
Xu C, Wu A, Yue Y (2012) Which is more effective in adolescent idiopathic scoliosis surgery: batroxobin, tranexamic acid or a combination? Arch Orthop Trauma Surg 132(1):25–31. doi:10.1007/s00402-011-1390-6
Wang Q, Liu J, Fan R et al (2013) Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J 22:2035–2038. doi:10.1007/s00586-013-2836-z
Raksakietisak M, Sathitkarnmanee B, Srisaen P et al (2015) Two Doses of tranexamic acid reduce blood transfusion in complex spine surgery: a prospective randomized study. Spine 40:E1257–E1263
Florentino-Pineda I, Thompson GH, Poe-Kochert C, Huang RP, Haber LL, Blakemore LC (2004) The effect of amicar on perioperative blood loss in idiopathic scoliosis: the results of a prospective, randomized double-blind study. Spine 29(3):233–238
Berenholtz SM, Pham JC, Garrett-Mayer E, Atchison CW, Kostuik JP et al. (2009) Effect of epsilon aminocaproic acid on red-cell transfusion requirements in major spinal surgery. Spine (Phila Pa 1976) 34: 2096–2103
Peters A, Verma K, Slobodyanyuk K et al (2015) Antifibrinolytics reduce blood loss in adult spinal deformity surgery: a prospective randomized controlled trial. Spine 40:E443–E449
Ortmann E, Besser MW, Klein AA (2013) Antifibrinolytic agents in current anaesthetic practice. Br J Anaesth 111(4):549–563. doi:10.1093/bja/aet154
K G, EGD T, JH M (2011) Normal haemostasis. In: Hoffbrand AV, Catovsky D, Tuddenham EGD (eds) Postgraduate haematology. Wiley-Blackwell, Oxford
U H, J H, V M, Colman RW, Hirsch J, Marder V, Clowes A, George J (2001) Hemostasis and thrombosis: basic principles and clinical practice. Lippincott Williams &Wilkins, Philadelphia
Dietrich W, Spath P, Zuhlsdorf M, et al. (2001) Anaphylactic reactions to aprotinin reexposure in cardiac surgery: relation to antiaprotinin immunoglobulin G and E antibodies. Anesthesiology 95:64–71 (discussion 5A–6A)
Eberle B, Mayer E, Hafner G et al (1998) High dose epsilon-aminocaproic acid versus aprotinin: antifibrinolytic therapy in first-time coronary operations. Ann Thorac Surg 65:667–673
Beierlein W, Scheule AM, Ziemer G (2000) Anaphylactic aprotinin reaction. Ann Thorac Surg 69:1298
Mangano DT, Tudor IC, Dietzel C, Ischemia Research G, Ischemia R, Education F (2006) The risk associated with aprotinin in cardiac surgery. New England J Med 354(4):353–365. doi:10.1056/NEJMoa051379
Mangano DT, Miao Y, Vuylsteke A, Tudor IC, Juneja R, Filipescu D, et al. (2007)Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA: the journal of the American Medical Association. 297(5):471–479. doi:10.1001/jama.297.5.471
Rao BH, Saxena N, Chauhan S et al (2000) Epsilon aminocaproic acid in paediatric cardiac surgery to reduce postoperative blood loss. Indian J Med Res 111:57–61
Vander Salm TJ, Kaur S, Lancey RA et al (1996) Reduction of bleeding after heart operations through the prophylactic use of epsilon-aminocaproic acid. J Thoracic Cardiovasc Surg 112:1098–1107
Williams GD, Bratton SL, Riley EC et al (1999) Efficacy of epsilon-aminocaproic acid in children undergoing cardiac surgery. J Cardiothorac Vasc Anesth 13:304–308
Yagi M, Hasegawa J, Nagoshi N, lizuka S, Kaneko S, et al. (2012) Does the intraoperative tranexamic Acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine (Phila Pa 1976) 37:1336–1342
Thompson GH, Florentino-Pineda I, Poe-Kochert C, Armstrong DG, Son-Hing (2008) The role of amicar in same-day anterior and posterior spinal fusion for idiopathic scoliosis. Spine (Phila Pa 1976) 33:2237–2242
Dhawale AA, Shah SA, Sponseller PD, Bastrom T, Neiss G et al. (2012) Are antifibrinolytics helpful in decreasing blood loss and transfusions during spinal fusion surgery in children with cerebral palsy scoliosis? Spine (Phila Pa 1976) 37:549–555
Jain A, Karas DJ, Skolasky RL, Sponseller PD (2014) Thromboembolic complications in children after spinal fusion surgery. Spine 39(16):1325–1329. doi:10.1097/BRS.0000000000000402
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors having conflict of interests.
Rights and permissions
About this article
Cite this article
Li, G., Sun, TW., Luo, G. et al. Efficacy of antifibrinolytic agents on surgical bleeding and transfusion requirements in spine surgery: a meta-analysis. Eur Spine J 26, 140–154 (2017). https://doi.org/10.1007/s00586-016-4792-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4792-x