Abstract
Purpose
To determine whether particulate debris is present in periprosthetic tissue from revised Dynesys® devices, and if present, elicits a biological tissue reaction.
Methods
Five Dynesys® dynamic stabilization systems consisting of pedicle screws (Ti alloy), polycarbonate-urethane (PCU) spacers and a polyethylene-terephthalate (PET) cord were explanted for pain and screw loosening after a mean of 2.86 years (1.9–5.3 years). Optical microscopy and scanning electron microscopy were used to evaluate wear, deformation and surface damage, and attenuated total reflectance Fourier transform infrared spectroscopy to assess surface chemical composition of the spacers. Periprosthetic tissue morphology and wear debris were determined using light microscopy, and PCU and PET wear debris by polarized light microscopy.
Results
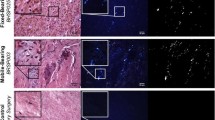
All implants had surface damage on the PCU spacers consistent with scratches and plastic deformation; 3 of 5 exhibited abrasive wear zones. In addition to fraying of the outer fibers of the PET cords in five implants, one case also evidenced cord fracture. The pedicle screws were unremarkable. Patient periprosthetic tissues around the three implants with visible PCU damage contained wear debris and a corresponding macrophage infiltration. For the patient revised for cord fracture, the tissues also contained large wear particles (>10 μm) and giant cells. Tissues from the other two patients showed comparable morphologies consisting of dense fibrous tissue with no inflammation or wear debris.
Conclusions
This is the first study to evaluate wear accumulation and local tissue responses for explanted Dynesys® devices. Polymer wear debris and an associated foreign-body macrophage response were observed in three of five cases.






Similar content being viewed by others
References
Yu S-W, Yang S-C, Ma C-H, Wu C-H, Yen C-Y, Tu Y-K (2012) Comparison of Dynesys posterior stabilization and posterior lumbar interbody fusion for spinal stenosis L4L5. Acta Orthop Belg 78:230–239
Di Silvestre M, Lolli F, Bakaloudis G, Parisini P (2010) Dynamic stabilization for degenerative lumbar scoliosis in elderly patients. Spine (Phila Pa 1976) 35:227–234. doi:10.1097/BRS.0b013e3181bd3be6
Schnake KJ, Schaeren S, Jeanneret B (2006) Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine (Phila Pa 1976) 31:442–449. doi:10.1097/01.brs.0000200092.49001.6e
Schwarzenbach O, Rohrbach N, Berlemann U (2010) Segment-by-segment stabilization for degenerative disc disease: a hybrid technique. Eur Spine J 19:1010–1020. doi:10.1007/s00586-010-1282-4
Stoll TM, Dubois G, Schwarzenbach O (2002) The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J 11(Suppl 2):S170–S178
Klockner C (2010) Long-term results of the Dynesys implant. Orthopade 39:559–564. doi:10.1007/s00132-009-1585-5
Schaeren S, Broger I, Jeanneret B (2008) Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine 33:E636–E642. doi:10.1097/BRS.0b013e31817d2435
Grob D, Benini A, Junge A, Mannion AF (2005) Clinical experience with the Dynesys semirigid fixation system for the lumbar spine: surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine 30:324–331
Wurgler-Hauri CC, Kalbarczyk A, Wiesli M, Landolt H, Fandino J (2008) Dynamic neutralization of the lumbar spine after microsurgical decompression in acquired lumbar spinal stenosis and segmental instability. Spine (Phila Pa 1976) 33:E66–E72. doi:10.1097/BRS.0b013e31816245c0
Kim CH, Chung CK, Jahng TA (2011) Comparisons of outcomes after single or multilevel dynamic stabilization: effects on adjacent segment. J Spinal Disord Tech 24:60–67. doi:10.1097/BSD.0b013e3181d4eb44
Bothmann M, Kast E, Boldt GJ, Oberle J (2008) Dynesys fixation for lumbar spine degeneration. Neurosurg Rev 31:189–196
Kocak T, Cakir B, Reichel H, Mattes T (2010) Screw loosening after posterior dynamic stabilization–review of the literature. Acta Chir Orthop Traumatol Cech 77:134–139
Lutz JA, Otten P, Maestretti G (2012) Late infections after dynamic stabilization of the lumbar spine with Dynesys. Eur Spine J 21:2573–2579. doi:10.1007/s00586-012-2366-0
Ko C-C, Tsai H-W, Huang W-C, Wu J-C, Chen Y-C, Shih Y-H, Chen H-C, Wu C-L, Cheng H (2010) Screw loosening in the Dynesys stabilization system: radiographic evidence and effect on outcomes. Neurosurg 28:E10. doi:10.3171/2010.3.FOCUS1052
Schilling C, Kruger S, Grupp TM, Duda GN, Blomer W, Rohlmann A (2011) The effect of design parameters of dynamic pedicle screw systems on kinematics and load bearing: an in vitro study. Eur Spine J 20:297–307. doi:10.1007/s00586-010-1620-6
Gedet P, Haschtmann D, Thistlethwaite PA, Ferguson SJ (2009) Comparative biomechanical investigation of a modular dynamic lumbar stabilization system and the Dynesys system. Eur Spine J 18:1504–1511. doi:10.1007/s00586-009-1077-7
Schmoelz W, Huber JF, Nydegger T, Dipl I, Claes L, Wilke HJ (2003) Dynamic stabilization of the lumbar spine and its effects on adjacent segments: an in vitro experiment. J Spinal Disord Tech 16:418–423
Kurtz SM, MacDonald D, Ianuzzi A, van Ooij A, Isaza J, Ross ER, Regan J (2009) The natural history of polyethylene oxidation in total disc replacement. Spine (Phila Pa 1976) 34:2369–2377. doi:10.1097/BRS.0b013e3181b20230
Ianuzzi A, Kurtz SM, Kane W, Shah P, Siskey R, van Ooij A, Bindal R, Ross R, Lanman T, Buttner-Janz K, Isaza J (2010) In vivo deformation, surface damage, and biostability of retrieved Dynesys systems. Spine 35:E1310–E1316. doi:10.1097/BRS.0b013e3181d6f84f
Shen M, Zhang K, Koettig P, Welch WC, Dawson JM (2011) In vivo biostability of polymeric spine implants: retrieval analyses from a United States investigational device exemption study. Eur Spine J 20:1837–1849. doi:10.1007/s00586-011-1812-8
Christenson EM, Anderson JM, Hiltner A (2004) Oxidative mechanisms of poly(carbonate urethane) and poly(ether urethane) biodegradation: in vivo and in vitro correlations. J Biomed Mater Res A 70:245–255. doi:10.1002/jbm.a.30067
Grammatopoulos G, Pandit H, Kamali A, Maggiani F, Glyn-Jones S, Gill HS, Murray DW, Athanasou N (2013) The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg Am 95:e81. doi:10.2106/JBJS.L.00775
Veruva SY, Lanman TH, Isaza JE, Macdonald DW, Kurtz SM, Steinbeck MJ (2014) UHMWPE wear debris and tissue reactions are reduced for contemporary designs of lumbar total disc replacements. Clin Orthop Relat Res (in press)
Baxter RM, Ianuzzi A, Freeman TA, Kurtz SM, Steinbeck MJ (2010) Distinct immunohistomorphologic changes in periprosthetic hip tissues from historical and highly crosslinked UHMWPE implant retrievals. J Biomed Mater Res A 95:68–78. doi:10.1002/jbm.a.32813
Cipriani E, Bracco P, Kurtz SM, Costa L, Zanetti M (2013) In-vivo degradation of poly(carbonate-urethane) based spine implants. Polym Degrad Stab 98:1225–1235. doi:10.1016/j.polymdegradstab.2013.03.005
Fisher J, Bell J, Barbour PS, Tipper JL, Matthews JB, Besong AA, Stone MH, Ingham E (2001) A novel method for the prediction of functional biological activity of polyethylene wear debris. Proc Inst Mech Eng H 215:127–132
Vanden Bossche L, Vanderstraeten G (2005) Heterotopic ossification: a review. J Rehabil Med 37:129–136. doi:10.1080/16501970510027628
McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J (2003) Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech 16:384–389
Guyer RD, McAfee PC, Banco RJ, Bitan FD, Cappuccino A, Geisler FH, Hochschuler SH, Holt RT, Jenis LG, Majd ME, Regan JJ, Tromanhauser SG, Wong DC, Blumenthal SL (2009) Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: five-year follow-up. Spine J 9:374–386. doi:10.1016/j.spinee.2008.08.007
Ingham E, Fisher J (2000) Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng H 214:21–37
Acknowledgments
We thank Eual A. Phillips for his help in performing the image analyses for this paper.
IRB approval
Yes.
Conflict of interest
This study was supported by a grant from the NIAMS (NIH R01 AR56264). One of the authors (SMK) is an employee and shareholder of Exponent, Inc, and institutional support for SMK is received as a Principal Investigator from Smith & Nephew; Stryker Orthopaedics; Zimmer Inc; Biomet; DePuy Synthes; Medtronic; Invibio; Stelkast; Formae; Kyocera Medical; Wright Medical Technology; CeramTec; DJO; Celanese; Aesculap; SpinalMotion, Inc; and Active Implants outside of the submitted work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neukamp, M., Roeder, C., Veruva, S.Y. et al. In vivo compatibility of Dynesys® spinal implants: a case series of five retrieved periprosthetic tissue samples and corresponding implants. Eur Spine J 24, 1074–1084 (2015). https://doi.org/10.1007/s00586-014-3705-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3705-0