Abstract
Purpose
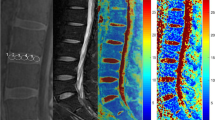
To evaluate intervertebral disc (IVD) degeneration and treatments, an objective diagnostic tool is needed. Recently, T2* relaxation time mapping was proposed as a technique to assess early IVD degeneration, yet the correlation with biochemical content and histological features has not been investigated previously. Our objective was to validate T2* mapping for disc degeneration by correlating this technique with accepted parameters of IVD degeneration.
Methods
Mildly and severely degenerated lumbar discs were obtained from an in vivo large animal study; two healthy goat spines were acquired as control. In total, 48 IVDs were analysed using T2-weighted MRI, T2* relaxation time mapping, biochemical assays, macroscopic and histological scoring. Correlations between variables were expressed with Spearman’s rho (ρ) coefficients.
Results
A complete range of degenerative grades were obtained (mean histological grade 2.2, range 0–6). A linear positive correlation was observed between T2* relaxation time and glycosaminoglycan content (ρ = 0.64, p < 0.001). T2* relaxation time decreased linearly with increasing degeneration as assessed with Pfirrmann scoring system (ρ = −0.67, p < 0.001), macroscopic (ρ = −0.33, p < 0.05) and histological (ρ = −0.45, p < 0.05) grading.
Conclusions
T2* mapping is an MRI technique for IVD evaluation which allows for measurements on a continuous scale thus minimising observer bias compared to grading systems. Although limited by a small sample size, this study showed a relatively good and linear correlation between T2* relaxation time and accepted parameters of disc degeneration. This suggests that T2* mapping is a promising tool to assess disc degeneration in clinical practice.




Similar content being viewed by others
References
Andersson GB (1999) Epidemiological features of chronic low-back pain. Lancet 354:581–585. doi:10.1016/S0140-6736(99)01312-4
Cheung KMC, Karppinen J, Chan D, Ho DWH, Song Y-Q, Sham P, Cheah KSE, Leong JCY, Luk KDK (2009) Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 34:934–940. doi:10.1097/BRS.0b013e3181a01b3f
Katz JN (2006) Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am 88(Suppl 2):21–24. doi:10.2106/JBJS.E.01273
Roughley PJ (2004) Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine 29:2691–2699
Adams MA, Roughley PJ (2006) What is intervertebral disc degeneration, and what causes it? Spine 31:2151–2161. doi:10.1097/01.brs.0000231761.73859.2c
Bansal PN, Stewart RC, Entezari V, Snyder BD, Grinstaff MW (2011) Contrast agent electrostatic attraction rather than repulsion to glycosaminoglycans affords a greater contrast uptake ratio and improved quantitative CT imaging in cartilage. Osteoarthr Cartil 19:970–976. doi:10.1016/j.joca.2011.04.004
Jazini E, Sharan AD, Morse LJ, Dyke JP, Aronowitz EB, Chen LKH, Tang SY (2012) Alterations in T2 relaxation magnetic resonance imaging of the ovine intervertebral disc due to nonenzymatic glycation. Spine 37:E209–E215. doi:10.1097/BRS.0b013e31822ce81f
Morgan S, Saifuddin A (1999) MRI of the lumbar intervertebral disc. Clin Radiol 54:703–723
Luoma K, Vehmas T, Riihimäki H, Raininko R (2001) Disc height and signal intensity of the nucleus pulposus on magnetic resonance imaging as indicators of lumbar disc degeneration. Spine 26:680–686
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26:1873–1878
Marinelli NL, Haughton VM, Muñoz A, Anderson PA (2009) T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine 34:520–524. doi:10.1097/BRS.0b013e318195dd44
Perry J, Haughton V, Anderson PA, Wu Y, Fine J, Mistretta C (2006) The value of T2 relaxation times to characterize lumbar intervertebral disks: preliminary results. AJNR Am J Neuroradiol 27:337–342
Wang Y-XJ, Zhao F, Griffith JF, Mok GSP, Leung JCS, Ahuja AT, Yuan J (2013) T1rho and T2 relaxation times for lumbar disc degeneration: an in vivo comparative study at 3.0-Tesla MRI. Eur Radiol 23:228–234. doi:10.1007/s00330-012-2591-2
Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, Elliott DM (2006) Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine 31:1253–1257. doi:10.1097/01.brs.0000217708.54880.51
Auerbach JD, Johannessen W, Borthakur A, Wheaton AJ, Dolinskas CA, Balderston RA, Reddy R, Elliott DM (2006) In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J 15(Suppl 3):S338–S344. doi:10.1007/s00586-006-0083-2
Blumenkrantz G, Zuo J, Li X, Kornak J, Link TM, Majumdar S (2010) In vivo 3.0-tesla magnetic resonance T1rho and T2 relaxation mapping in subjects with intervertebral disc degeneration and clinical symptoms. Magn Reson Med 63:1193–1200. doi:10.1002/mrm.22362
Zhang W, Ma X, Wang Y, Zhao J, Zhang X, Gao Y, Li S (2014) Assessment of apparent diffusion coefficient in lumbar intervertebral disc degeneration. Eur Spine J. doi:10.1007/s00586-014-3285-z
Niinimäki J, Korkiakoski A, Ojala O, Karppinen J, Ruohonen J, Haapea M, Korpelainen R, Natri A, Tervonen O (2009) Association between visual degeneration of intervertebral discs and the apparent diffusion coefficient. Magn Reson Imaging 27:641–647. doi:10.1016/j.mri.2008.10.005
Antoniou J, Demers CN, Beaudoin G, Goswami T, Mwale F, Aebi M, Alini M (2004) Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Magn Reson Imaging 22:963–972. doi:10.1016/j.mri.2004.02.011
Beattie PF, Morgan PS, Peters D (2008) Diffusion-weighted magnetic resonance imaging of normal and degenerative lumbar intervertebral discs: a new method to potentially quantify the physiologic effect of physical therapy intervention. J Orthop Sports Phys Ther 38:42–49. doi:10.2519/jospt.2008.2631
Kealey SM, Aho T, Delong D, Barboriak DP, Provenzale JM, Eastwood JD (2005) Assessment of apparent diffusion coefficient in normal and degenerated intervertebral lumbar disks: initial experience. Radiology 235:569–574. doi:10.1148/radiol.2352040437
Hoppe S, Quirbach S, Mamisch TC, Krause FG, Werlen S, Benneker LM (2012) Axial T2 mapping in intervertebral discs: a new technique for assessment of intervertebral disc degeneration. Eur Radiol 22:2013–2019. doi:10.1007/s00330-012-2448-8
Welsch GH, Trattnig S, Paternostro-Sluga T, Bohndorf K, Goed S, Stelzeneder D, Mamisch TC (2011) Parametric T2 and T2* mapping techniques to visualize intervertebral disc degeneration in patients with low back pain: initial results on the clinical use of 3.0 Tesla MRI. Skeletal Radiol 40:543–551. doi:10.1007/s00256-010-1036-8
Biercevicz AM, Murray MM, Walsh EG, Miranda DL, Machan JT, Fleming BC (2013) T2* MR relaxometry and ligament volume are associated with the structural properties of the healing ACL. J Orthop Res. doi:10.1002/jor.22563
Krause FG, Klammer G, Benneker LM, Werlen S, Mamisch TC, Weber M (2010) Biochemical T2* MR quantification of ankle arthrosis in pes cavovarus. J Orthop Res 28:1562–1568. doi:10.1002/jor.21192
Mamisch TC, Hughes T, Mosher TJ, Mueller C, Trattnig S, Boesch C, Welsch GH (2012) T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol 41:287–292. doi:10.1007/s00256-011-1171-x
Stelzeneder D, Shetty AA, Kim S-J, Trattnig S, Domayer SE, Shetty V, Bilagi P (2013) Repair tissue quality after arthroscopic autologous collagen-induced chondrogenesis (ACIC) assessed via T2* mapping. Skeletal Radiol 42:1657–1664. doi:10.1007/s00256-013-1708-2
Ellingson AM, Mehta H, Polly DW, Ellermann J, Nuckley DJ (2013) Disc degeneration assessed by quantitative T2* (T2 star) correlated with functional lumbar mechanics. Spine. doi:10.1097/BRS.0b013e3182a59453
Hoogendoorn RJW, Helder MN, Kroeze RJ, Bank RA, Smit TH, Wuisman PIJM (2008) Reproducible long-term disc degeneration in a large animal model. Spine 33:949–954. doi:10.1097/BRS.0b013e31816c90f0
Hoogendoorn RJW, Wuisman PIJM, Smit TH, Everts VE, Helder MN (2007) Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine 32:1816–1825. doi:10.1097/BRS.0b013e31811ebac5
Norcross JP, Lester GE, Weinhold P, Dahners LE (2003) An in vivo model of degenerative disc disease. J Orthop Res 21:183–188. doi:10.1016/S0736-0266(02)00098-0
Detiger SEL, Hoogendoorn RJW, van der Veen AJ, Van Royen BJ, Helder MN, Koenderink GH, Smit TH (2013) Biomechanical and rheological characterization of mild intervertebral disc degeneration in a large animal model. J Orthop Res 31:703–709. doi:10.1002/jor.22296
Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB (1990) Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine 15:411–415
Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS (2005) A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14
Paul CPL, Zuiderbaan HA, Zandieh Doulabi B, van der Veen AJ, van de Ven PM, Smit TH, Helder MN, Van Royen BJ, Mullender MG (2012) Simulated-physiological loading conditions preserve biological and mechanical properties of caprine lumbar intervertebral discs in ex vivo culture. PLoS ONE 7:e33147. doi:10.1371/journal.pone.0033147
Detiger SEL, Hoogendoorn RJW, Mevorat Kaplan K, van Royen BJ, Smit TH, Yayon A, Helder MN. Observations after SVF injection into goat intervertebral discs. Presented at International Federation for Adipose Therapeutics and Science (IFATS) 11th annual meeting, New York City, USA, 21–23 Nov 2013, p 71
Acknowledgments
The authors would like to thank Klaas-Walter Meyer and Paul Sinnige for their assistance with the surgeries and Francisca Galindo Garre for her help with the statistical analysis. Funding for this study was provided by the European Commission (FP7 project “NPmimetic”; Grant number #246351).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Detiger, S.E.L., Holewijn, R.M., Hoogendoorn, R.J.W. et al. MRI T2* mapping correlates with biochemistry and histology in intervertebral disc degeneration in a large animal model. Eur Spine J 24, 1935–1943 (2015). https://doi.org/10.1007/s00586-014-3498-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3498-1