Abstract
Objectives
The pathophysiology of radiculopathy associated with lumbar spinal stenosis and lumbar disc herniation is incompletely understood. The goal of the present study was to establish a chronic spinal nerve root compression model that can mimic lumbar disc herniation or spinal stenosis using silicone tube compression. We also try to link the pathology changes of damaged nerve root with the reaction of microglia in spinal cord in same rat at different time points.
Methods
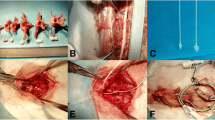
Thirty rats were used in this study. The L5 nerve roots (dorsal and ventral) were exposed by hemilaminectomy; the diameter of the L5 nerve root was measured at the 2 mm proximal from the dorsal root ganglia. The dorsal and ventral nerve roots of L5 were compressed using a silicone tube, and the sham group was only exposed dorsal and ventral roots of L5. Five rats from the sham group were perfused at 8 days after surgery, and 25 rats from the model groups were perfused at 3, 8, 12, 45 days, and 5 months after surgery, each model group was composed of 5 rats according to the time point. The L5 spinal cord segments and nerve root that compressed by silicone tube were harvested from the same rat. Microglia and neuron in the spinal cord were stained by immunohistochemistry, and the nerve root was shown by electron microscope.
Results
In sham-operated rat, the arrangement of axon and myelin sheath is normal, the ventral root is mainly composed of large axon (>6 μm) and it is composed of 46.3 % of all the axons of the ventral root; the average myelin thickness of large axon is 1.86 μm; the dorsal root is mainly composed of medium (2–3.9 or 4–5.9 μm) axons and they are composed of 79.1 % of all the axons of the dorsal root; the average myelin thickness of this category is 0.94 or 1.55 μm. The average myelin thickness of large axon in ventral root reduced to 0.97 and 1.19 μm from more than 1.86 μm after compression for 3 and 8 days separately. Most of myelin sheath disappeared after 12 days of compression; the myelin sheath was partly restored at 45 days after compression which the myelin sheath thickness of large axons in ventral root was 0.47 μm. The medium category in dorsal root reduced to 0.59 or 0.72 μm from 0.94 μm, and 1.55 μm after compression for 3 days (p < 0.05 to p < 0.0001). The medium category axon in dorsal root is also 0.47 μm after compression for 45 days (p ≤ 0.0001). The myelin sheath was almost totally restored at the 5 months of compression; the myelin sheath thickness returned to normal and the axons were intact in structure under EM. The number of Iba1-positive microglia increased by 18.69, 40.44, and 18.49 % after compression for 3, 8, and 12 days separately in the ipsilateral dorsal horn and 21.26, 32.15, 22.87 % in ventral horns, and the activation of microglia was also prominent in contralateral sides of the dorsal and ventral horn at 8 days time point. The microglia cell reconverted to resting status after compression for 45 days or 5 months.
Conclusion
The chronic spinal nerve root compression with silicone tube produces a recoverable damage to nerve root, which produces recoverable microglial activation in the spinal cord. These results demonstrated that the chronic spinal nerve root compression with silicone tube could mimic the pathological changes of lumbar spinal stenosis or lumbar disc herniation.








Similar content being viewed by others
References
Calingasan NY, Huang PL, Chun HS, Fabian A, Gibson GE (2000) Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J Neuropathol Exp Neurol 59:207–217
Chaudhry V, Glass JD, Griffin JW (1992) Wallerian degeneration in peripheral nerve disease. Neurol Clin 10:613–627
Goodrum JF, Earnhardt T, Goines N, Bouldin TW (1994) Fate of myelin lipids during degeneration and regeneration of peripheral nerve: an autoradiographic study. J Neurosci 14:357–367
Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O (2004) Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol 187:500–508
Gupta R, Steward O (2003) Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol 461:174–186
Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y (2000) Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem 75:965–972
Hollingworth W, Dixon AK, Todd CJ, Bell MI, Antoun NM, Arafat Q, Girling S, Karia KR, Laing RJ (1998) Self reported health status and magnetic resonance imaging findings in patients with low back pain. Eur Spine J 7:369–375
Igarashi T, Kikuchi S, Shubayev V, Myers RR (2000) 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine (Phila Pa 1976) 25:2975–2980
Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Ozawa T, Saito T, Moriya H, Takahashi K (2007) Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine (Phila Pa 1976) 32:159–167
Iwashita Y, Blakemore WF (2000) Areas of demyelination do not attract significant numbers of Schwann cells transplanted into normal white matter. Glia 31:232–240
Kallakuri S, Takebayashi T, Ozaktay AC, Chen C, Yang S, Wooley PH, Cavanaugh JM (2005) The effects of epidural application of allografted nucleus pulposus in rats on cytokine expression, limb withdrawal and nerve root discharge. Eur Spine J 14:956–964
Karuppagounder SS, Shi Q, Xu H, Gibson GE (2007) Changes in inflammatory processes associated with selective vulnerability following mild impairment of oxidative metabolism. Neurobiol Dis 26:353–362
Kobayashi S, Yoshizawa H (2002) Effect of mechanical compression on the vascular permeability of the dorsal root ganglion. J Orthop Res 20:730–739
Kobayashi S, Yoshizawa H, Hachiya Y, Ukai T, Morita T (1993) Vasogenic edema induced by compression injury to the spinal nerve root. Distribution of intravenously injected protein tracers and gadolinium-enhanced magnetic resonance imaging. Spine (Phila Pa 1976) 18:1410–1424
Kobayashi S, Yoshizawa H, Yamada S (2004) Pathology of lumbar nerve root compression. Part 1: intraradicular inflammatory changes induced by mechanical compression. J Orthop Res 22:170–179
Kobayashi S, Yoshizawa H, Yamada S (2004) Pathology of lumbar nerve root compression. Part 2: morphological and immunohistochemical changes of dorsal root ganglion. J Orthop Res 22:180–188
Lacroix-Fralish ML, Tawfik VL, Tanga FY, Spratt KF, DeLeo JA (2006) Differential spinal cord gene expression in rodent models of radicular and neuropathic pain. Anesthesiology 104:1283–1292
Larsen JO (1998) Stereology of nerve cross sections. J Neurosci Methods 85:107–118
Ludwin SK, Maitland M (1984) Long-term remyelination fails to reconstitute normal thickness of central myelin sheaths. J Neurol Sci 64:193–198
Mackinnon SE, Dellon AL, Hudson AR, Hunter DA (1986) Chronic human nerve compression—a histological assessment. Neuropathol Appl Neurobiol 12:547–565
Murata Y, Nannmark U, Rydevik B, Takahashi K, Olmarker K (2006) Nucleus pulposus-induced apoptosis in dorsal root ganglion following experimental disc herniation in rats. Spine (Phila Pa 1976) 31:382–390
Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S, Kurihara T (2001) Neurotrophin secretion from cultured microglia. J Neurosci Res 65:322–331
Nakajima K, Kohsaka S (2001) Microglia: activation and their significance in the central nervous system. J Biochem 130:169–175
Olmarker K, Iwabuchi M, Larsson K, Rydevik B (1998) Walking analysis of rats subjected to experimental disc herniation. Eur Spine J 7:394–399
Olmarker K, Nordborg C, Larsson K, Rydevik B (1996) Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine (Phila Pa 1976) 21:411–414
Olmarker K, Rydevik B, Holm S (1989) Edema formation in spinal nerve roots induced by experimental, graded compression. An experimental study on the pig cauda equina with special reference to differences in effects between rapid and slow onset of compression. Spine (Phila Pa 1976) 14:569–573
Omarker K, Myers RR (1998) Pathogenesis of sciatic pain: role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain 78:99–105
Porter RW, Hibbert C, Evans C (1984) The natural history of root entrapment syndrome. Spine (Phila Pa 1976) 9:418–421
Saal JA, Saal JS (1989) Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine (Phila Pa 1976) 14:431–437
Salzer JL (2008) Switching myelination on and off. J Cell Biol 181:575–577
Sekiguchi M, Kikuchi S, Myers RR (2004) Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine (Phila Pa 1976) 29:1105–1111
Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K (2001) Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem 78:1339–1349
Takayama B, Sekiguchi M, Yabuki S, Fujita I, Shimada H, Kikuchi S (2008) Gene expression changes in dorsal root ganglion of rat experimental lumber disc herniation models. Spine (Phila Pa 1976) 33:1829–1835
Tomita K, Kubo T, Matsuda K, Yano K, Tohyama M, Hosokawa K (2007) Myelin-associated glycoprotein reduces axonal branching and enhances functional recovery after sciatic nerve transection in rats. Glia 55:1498–1507
Vucetic N, Astrand P, Guntner P, Svensson O (1999) Diagnosis and prognosis in lumbar disc herniation. Clin Orthop Relat Res 361:116–122
Watkins LR, Maier SF (2003) Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov 2:973–985
Winkelstein BA, DeLeo JA (2004) Mechanical thresholds for initiation and persistence of pain following nerve root injury: mechanical and chemical contributions at injury. J Biomech Eng 126:258–263
Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA (2001) Quantification of neural tissue injury in a rat radiculopathy model: comparison of local deformation, behavioral outcomes, and spinal cytokine mRNA for two surgeons. J Neurosci Methods 111:49–57
Winkelstein BA, Weinstein JN, DeLeo JA (2002) The role of mechanical deformation in lumbar radiculopathy: an in vivo model. Spine (Phila Pa 1976) 27:27–33
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, F., Wei, Y., Chen, Y. et al. A rat model for chronic spinal nerve root compression. Eur Spine J 23, 435–446 (2014). https://doi.org/10.1007/s00586-013-2990-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-013-2990-3