Abstract
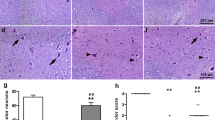
The aim of this experimental study was to investigate the possible protective effect of dexmedetomidine (DEX) on traumatic spinal cord injury (SCI). Twenty-two New Zealand rabbits were divided into three groups: sham (no drug or operation, n = 6), Control [SCI + single dose of 1 mL saline intraperitoneally (i.p), after trauma; n = 8] and DEX (SCI + 1 μg/kg dexmedetomidine in 1 mL, i.p, after trauma, n = 8). Laminectomy was performed at T10 and balloon angioplasty catheter was applied extradurally. Four and 24 h after surgery, rabbits were evaluated by an independent observer according to the Tarlov scoring system. Blood, cerebrospinal fluid (CSF), tissue samples from spinal cord were taken for biochemical and histopathological evaluations. After 4 h of SCI, all animals in control or DEX treated groups became paraparesic. On the other hand, 24 h after SCI, partial improvements were observed in both control and DEX treated groups. Traumatic SCI leads to increase in the lipid peroxidation and decreases enzymatic or nonenzymatic endogenous antioxidative defense systems. Again, SCI leads to apoptosis in spinal cord. DEX treatment slightly prevented lipid peroxidation and augmented endogenous antioxidative defense systems in CSF or spinal cord tissue, but failed to prevent apoptosis or neurodeficit after traumatic SCI. Therefore, it could be suggested that treatment with dexmedetomidine does not produce beneficial results in SCI.

Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Anderson DK, Hall ED (1993) Pathophysiology of spinal cord trauma. Ann Emerg Med 22:987–992. doi:10.1016/S0196-0644(05)82739-8
Ates O, Cayli SR, Altinoz E, Gurses I, Yucel N, Kocak A, Yologlu S, Turkoz Y (2006) Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta Pharmacol Sin 27:1317–1325. doi:10.1111/j.1745-7254.2006.00416.x
Ates O, Cayli SR, Gurses I, Turkoz Y, Tarim O, Cakir CO, Kocak A (2007) Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J Clin Neurosci 14:658–665. doi:10.1016/j.jocn.2006.03.023
Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE (1997) Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res 765:283–290. doi:10.1016/S0006-8993(97)00573-8
Blanchard B, Pompon D, Ducrocq C (2000) Nitrosation of melatonin by nitric oxide and peroxynitrite. J Pineal Res 29:184–192. doi:10.1034/j.1600-079X.2000.290308.x
Beutler E, Dubon O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 6:882–888
Cayli SR, Ates O, Karadag N, Altinoz E, Yucel N, Yologlu S, Kocak A, Cakir CO (2006) Neuroprotective effect of etomidate on functional recovery in experimental spinal cord injury. Int J Dev Neurosci 24:233–239. doi:10.1016/j.ijdevneu.2006.04.003
Cormack JR, Orme RM, Costello TG (2005) The role of a2-agonists in neurosurgery. J Clin Neurosci 12:375–378. doi:10.1016/j.jocn.2004.06.008
Cosar M, Eser O, Fidan H, Sahin O, Buyukbas S, Ela Y, Yagmurca M, Ozen OA (2009) The neuroprotective effect of dexmedetomidine in the hippocampus of rabbits after subarachnoid hemorrhage. Surg Neurol 71:54–59
Fehlings MG, Baptiste DC (2005) Current status of clinical trials for acute spinal cord injury. Injury 36:113–122. doi:10.1016/j.injury.2005.06.022
Flohe L, Otting F (1984) Superoxide dismutase assays. Methods Enzymol 105:93–104. doi:10.1016/S0076-6879(84)05013-8
Ha KY, Kim YH, Rhyu KW, Kwon SE (2008) Pregabalin as a neuroprotector after spinal cord injury in rats. Eur Spine J 17:864–872. doi:10.1007/s00586-008-0653-6
Hall ED (1993) Lipid peroxidants in acute central nervous system injury. Ann Emerg Med 22:1022–1027. doi:10.1016/S0196-0644(05)82745-3
Hayashi K, Noguchi N, Niki E (1995) Action of nitric oxide as an antioxidant against oxidation of soybean phosphatidylcholine liposomal membranes. FEBS Lett 370:37–40. doi:10.1016/0014-5793(95)00786-9
Jain SK, McVie R, Duett J, Herbst JJ (1989) Erythrocyte membrane lipid peroxidase and glycolylated hemoglobin in diabetes. Diabetes 38:1539–1543. doi:10.2337/diabetes.38.12.1539
Janke EL, Sarma S (2006) Dexmedetomidine and neuroprotection. Seminars in Anesthesia. Perioper Med Pain 25:71–76. doi:10.1053/j.sane.2006.02.002
Jessup W, Mohr D, Gieseg SP, Dean RT, Stocker R (1992) The participation of nitric oxide in cell free and its restriction of macrophage-mediated oxidation of low-density lipoprotein. Biochim Biophys Acta 1180:73–82
Kanter M, Coskun O, Kalayci M, Buyukbas S, Cagavi F (2006) Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum Exp Toxicol 25:127–133. doi:10.1191/0960327106ht608oa
Kaynar MY, Hanci M, Kuday C, Belce A, Gumustas K, Kokoglu E (1994) Changes in the activity of antioxidant enzymes (SOD, GPX, CAT) after experimental spinal cord injury. Tokushima J Exp Med 41:133–136
Kurihara M (1985) Role of monoamines in experimental spinal cord injury in rats. Relationship between Na + −K + −ATPase and lipid peroxidation. J Neurosurg 62:743–749
Liu JB, Tang TS, Yang HL (2006) Antioxidation of quercetin against spinal cord injury in rats. Chin J Traumatol 9:303–307
Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, Maze M (2004) Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol 11:87–97. doi:10.1016/j.ejphar.2004.08.044
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71. doi:10.1006/niox.2000.0319
Nogues MR, Giralt M, Romeu M, Mulero M, Sanchez-Martos V, Rodriguez E, Acuna-Castroviejo D, Mallol J (2006) Melatonin reduces oxidative stress in erythrocytes and plasma of senescence-accelerated mice. J Pineal Res 41:142–149. doi:10.1111/j.1600-079X.2006.00344.x
Omaye ST, Turnbul JD, Savberlich HE (1979) Ascorbic acid analysis II. Determination after derivatisation with 2.2. dinitrophenylhidrazine. Selected methods for determination of ascorbic acid in animal cells tissues and fluids. In: McCormick DB, Wright LD (eds) Methods in Enzymology, vol 62. Academic Press, New York, pp 7–8
Ozdemir M, Cengiz SL, Gurbilek M, Ogun TC, Ustun ME (2005) Effects of magnesium sulfate on spinal cord tissue lactate and malondialdehyde levels after spinal cord trauma. Magnes Res 18:170–174
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Papakostas JC, Matsagas MI, Toumpoulis IK, Malamou-Mitsi VD, Papa LS, Gkrepi C, Anagnostopoulos CE, Kappas AM (2006) Evolution of spinal cord injury in a porcine model of prolonged aortic occlusion. J Surg Res 133:159–166. doi:10.1016/j.jss.2005.10.007
Paris A, Mantz J, Tonner PH, Hein L, Brede M, Gressens P (2006) The effects of dexmedetomidine on perinatal excitotoxic brain injury are mediated by the alpha2A-adrenoceptor subtype. Anesth Analg 102:456–461. doi:10.1213/01.ane.0000194301.79118.e9
Samantaray S, Sribnick EA, Das A, Knaryan VH, Matzelle DD, Yallapragada AV, Reiter RJ, Ray SK, Banik NL (2007) Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. J Pineal Res 44:348–357. doi:10.1111/j.1600-079X.2007.00534.x
Suzuki I, Katoh N (1990) A simple and cheap method for measuring serum vitamin A in cattle using spectrophototmeter. Jpn J Vet Sci 52:1281–1283
Tator CH, Fehlings MG (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75:15–26
Taysi S, Koc M, Buyukokuroglu ME, Altinkaynak K, Sahin YN (2003) Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J Pineal Res 34:173–177. doi:10.1034/j.1600-079X.2003.00024.x
Tsifansky MD, Schmitt CG, Muñoz RA (2007) Dexmedetomidine: do we know enough? Pediatr Crit Care Med 8:492–493
Tsutsumi S, Ueta T, Shiba K (2006) Effects of the Second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury-results in spinal injuries center. Spine 31:2992–2997. doi:10.1097/01.brs.0000250273.28483.5c
Yoshino S, Yone K (1998) Role of norepinephrine and excitatory amino acids in edema of the spinal cord after experimental compression injury in rats. J Orthop Sci 3:54–59. doi:10.1007/s007760050021
Acknowledgments
This work was supported by Afyon Kocatepe University Scientific Research Fund (Project No: 051.TIP.17) and it was presented as e-poster in Spineweek 2008 (EuroSpine) in Geneva, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aslan, A., Cemek, M., Eser, O. et al. Does dexmedetomidine reduce secondary damage after spinal cord injury? An experimental study. Eur Spine J 18, 336–344 (2009). https://doi.org/10.1007/s00586-008-0872-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-008-0872-x