Abstract
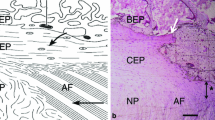
Introduction Spondylolytic spondylolisthesis is an osseous discontinuity of the vertebral arch that predominantly affects the fifth lumbar vertebra. Biomechanical factors are closely related to the condition. An immunohistochemical investigation of lysis-zone tissue obtained from patients with isthmic spondylolisthesis was performed to determine the molecular composition of the lysis-zone tissue and enable interpretation of the mechanical demands to which the tissue is subject. Methods: During surgery, the tissue filling the spondylytic defects was removed from 13 patients. Twelve spondylolistheses were at the L5/S1 level with slippage being less than Meyerding grade II. Samples were methanol fixed, decalcified and cryosectioned. Sections were labelled with a panel of monoclonal antibodies directed against collagens, glycosaminoglycans and proteoglycans. Results: The lysis-zone tissue had an ordered collagenous structure with distinct fibrocartilaginous entheses at both ends. Typically, these had zones of calcified and uncalcified fibrocartilage labelling strongly for type II collagen and aggrecan. Labelling was also detected around bony spurs that extended from the enthesis into the lysis-zone. The entheses also labelled for types I, III and VI collagens, chondroitin four and six sulfate, keratan and dermatan sulfate, link protein, versican and tenascin. Conclusions: Although the gap filled by the lysis tissue is a pathological feature, the tissue itself has hallmarks of a normal ligament—i.e. fibrocartilaginous entheses at either end of an ordered collagenous fibre structure. The fibrocartilage is believed to dissipate stress concentration at the hard/soft tissue boundary. The widespread occurrence of molecules typical of cartilage in the attachment of the lysis tissue, suggests that compressive and shear forces are present to which the enthesis is adapted, in addition to the expected tensile forces across the spondylolysis. Such a combination of tensile, shear and compressive forces must operate whenever there is any opening or closing of the spondylolytic gap.


Similar content being viewed by others
References
Bareggi R, Grill V, Zweyer M, Narducci P, Forabosco A (1994) A quantitative study on the spatial and temporal ossification patterns of vertebral centra and neural arches and their relationship to the fetal age. Ann Anat 176:311–317
Benjamin M, McGonagle D (2001) The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat 199:503–526
Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR (2002) The skeletal attachment of tendons—tendon entheses. Comp Biochem Phys A Mol Integr Physiol 133:931–945
Bono CM (2004) Low-back pain in athletes. J Bone Joint Surg Am 86A:382–396
Boszczyk BM, Boszczyk AA, Putz R, Büttner A, Benjamin M, Milz S (2001) An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine 26:E338–E343
Boszczyk AA, Boszczyk BM, Putz R, Benjamin M, Milz S (2003) Expression of a wide range of fibrocartilage molecules at the enthesis of the alar ligaments—possible antigenic targets of rheumatoid arthritis. J Rheumatol 30:1420–1425
Burr DB, Schaffler MB, Yang KH, Lukoschek M, Sivaneri N, Blaha JD, Radin EL (1989) Skeletal change in response to altered strain environments: is woven bone a response to elevated strain? Bone 10:223–233
Calabro A, Hascall VC, Caterson B (1992) Monoclonal antibodies directed against epitopes within the core protein structure of the large aggregating proetoglycan (aggrecan) from the swarm rat chondrosarcoma. Arch Biochem Biophys 298:349–360
Caterson B, Christner JE, Baker JR (1983) Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulphate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem 258:8848–8854
Caterson B, Christner JE, Baker JR, Couchman JR (1985) Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc 44:386–393
Chen CH, Chen WJ, Shih CH, Yang CY, Liu SJ, Lin PY (2003) Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: a biomechanical and histologic study in rabbits. Arthroscopy 19:290–296
Chiari H (1892) Die Ätiologie und Genese der sogenannten spondylolisthesis lumbo-sacralis. Z Heilk 13:199–262
Cullinane DM, Salisbury KT, Alkhiary Y, Eisenberg S, Gerstenfeld L, Einhorn TA (2003) Effects of the local mechanical environment on vertebrate tissue differentiation during repair: does repair recapitulate development? J Exp Biol 206:2459–2471
Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin Orthop 355(Suppl):S7–S21
Farfan HF, Osteria V, Lamy C (1975) The mechanical etiology of spondylolysis and spondylolisthesis. Clin Orthop 117:40–55
Fujioka H, Thakur R, Wang GJ, Mizuno K, Balian G, Hurwitz SR (1998) Comparison of surgically attached and non-attached repair of the rat achilles tendon-bone interface. Cellular organization and type X collagen expression. Connect Tissue Res 37:205–218
Gao J, Messner K, Ralphs JR, Benjamin M (1996) An immunohistochemical study of enthesis development in the medial collateral ligament of the rat knee joint. Anat Embryol 194:399–406
Haukipuro K, Keränen N, Koivisto E, Lindholm R, Norio R, Punto L (1978) Familial occurrence of lumbar spondylolysis and spondylolisthesis. Clin Genet 13:471–476
Heggeness MH, Esses SI, Mody DR (1993) A histologic study of lumbar pseudarthrosis. Spine 18:1016–1020
Hessle H, Engvall E (1984) Type VI collagen. Studies on its localization, structure, and biosynthetic form with monoclonal antibodies. J Biol Chem 259:3955–3961
Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H (1986) Characterization of the antibody response in mice with type II collagen-induced arthritis using monoclonal anti-type II collagen antibodies. Arth Rheum 29:400–410
Hutton WC, Stott JR, Cyron BM (1977) Is spondylolysis a fatigue fracture? Spine 2:202–209
Ikata T, Miyake R, Katoh S, Morita T, Murase M (1996) Pathogenesis of sports related spondylolisthesis in adolescents radiographic and magnetic resonance imaging study. Am J Sports Med 24:94–98
Inoue H, Ohmori K, Ishida Y, Suzuki K, Tanaka K, Murakami S (1998) Finite element analysis of the lower lumbar neural arch under facet loading. J Spinal Disord 11:241–247
Jackson RP, Phipps T, Hales C, Surber J (2003) Pelvic lordosis and alignment in spondylolisthesis. Spine 28:151–160
Kajiura K, Katoh S, Sairyo K, Ikata T, Goel VK, Murakami R (2001) Slippage mechanism of paediatric spondylolysis—biomechanical study using immature calf spines. Spine 26:2208–2213
Kilian HF (1853) De spondylolisthesi, gravissimae pelvangustiae causa nuper detecta. Commentatio anatomico-obstetricia. Bonn
Konz RJ, Goel VK, Grobler LJ, Grosland NM, Spratt KF, Scifert JL, Sairo K (2001) The pathomechanism of spondylolytic spondylolisthesis in immature primate lumbar spines—in vitro and finite element assessments. Spine 26:E38–E49
Lazennec JY, Laudet CG, Guerin-Surville H, Roy-Camille R, Saillant G (1997) Dynamic anatomy of the acetabulum: an experimental approach and surgical implications. Surg Radiol Anat 19:23–30
Loboa EG, Beaupre GS, Carter DR (2001) Mechanobiology of initial pseudarthrosis formation with oblique fractures. J Orthop Res 19:1067–1072
Lonstein JE (1999) Spondylolisthesis in children—cause, natural history and management. Spine 24:2640–2648
Löhe F, Eckstein F, Sauer T, Putz R (1996) Structure, strain and function of the transverse acetabular ligament. Acta Anat (Basel) 157:315–323
Major NM, Helms CA, Richardson WJ (1999) MR imaging of fibrocartilaginous masses arising on the margins of spondylolysis defects. Am J Roentgenol 173:673–676
Marty C, Boisaubert B, Descamps H, Montigny JP, Hecquet J, Legaye J, Duval-Beaupère (2002) The sagittal anatomy of the sacrum among young adults, infants, and spondylolisthesis patients. Eur Spine J 11:119–125
McTimoney CA, Micheli LJ (2003) Current evaluation and management of spondylolysis and spondylolisthesis. Curr Sports Med Rep 2:41–46
Milz S, Valassis G, Büttner A, Maier M, Putz R, Ralphs JR, Benjamin M (2001) Fibrocartilage in the transverse ligament of the human acetabulum. J Anat 198:223–228
Milz S, Schlüter T, Putz R, Moriggl B, Ralphs JR, Benjamin M (2001) Fibrocartilage in the transverse ligament of the human atlas. Spine 26:1765–1771
Moriggl B, Jax P, Milz S, Büttner A, Benjamin M (2001) Fibrocartilage at the entheses of the suprascapular (superior transverse scapular) ligament of man—a ligament spanning two regions of a single bone. J Anat 199:539–545
Mosimann P (1961) Die histologie der spondylolyse. Arch Orthop Unfallchir 53:264–285
Newell RL (1995) Spondylolysis—an historical review. Spine 20:1950–1956
Oguma H, Murakami G, Takahashi-Iwanaga H, Aoki M, Ishii S (2001) Early anchoring collagen fibers at the bone-tendon interface are conducted by woven bone formation: light microscope and scanning electron microscope observation using a canine model. J Orthop Res 19:873–880
Reichmann S (1971) The postnatal development of form and orientation of the lumbar intervertebral joint surfaces. Z Anat Entwicklungsgesch 133:102–123
Robert (1855) Eine eigenthümliche angeborene lordose, wahrscheinlich bedingt durch eine verschiebung des körpers des letzten lendenwirbels auf die vordere fläche des ersten kreuzbeinwirbels (Spondylolisthesis Kilian), nebst bemerkungen über die mechanik dieser beckenformation. Monatsschr f Geburtsk 5:81–94
Rosenberg NJ, Bargar WL, Friedman B (1981) The incidence of spondylolysis and spondylolisthesis in nonambulatory patients. Spine 6:35–38
Rufai A, Benjamin M, Ralphs JR (1992) Development and ageing of phenotypically distinct fibrocartilages associated with the rat achilles tendon. Anat Embryol 186:611–618
Sairo K, Katoh S, Sakamaki T, Inoue M, Komatsubara S, Ogawa T, Sano T, Goel VK, Yasui N (2004) Vertebral forward slippage in immature lumbar spine occurs following epiphyseal separation and its occurrence is unrelated to disc degeneration. Spine 29:524–527
Sakamaki T, Katoh S, Sairyo K (2002) Normal and spondylolytic pediatric spine movements with reference to instantaneous axis of rotation. Spine 27:141–145
Schneider H (1956) Zur struktur der sehnenansatzzonen. Z Anat 119:431–456
Schreiber A, Willert HG (1970) Zur pathogenese der spondylolyse. Verhdl Dtsch Orthop Traumat Ges 56:333
Stewart T (1953) The age incidence of neural arch defects in alaskan natives, considered from the standpoint of etiology. J Bone Joint Surg 35-A:937–950
Sys J, Michielsen J, Bracke P, Martens M, Verstreken J (2001) Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J 10:498–504
Taillard WF (1976) Etiology of spondylolisthesis. Clin Orthop 117:30–39
Töndury G (1958) Entwicklungsgeschichte und fehlbildungen der wirbelsäule. In: Junghans H (ed) Die Wirbelsäule in Forschung und Praxis. Stuttgart: Hippokrates 39–46
Uhthoff HK, Seki M, Backman DS, Trudel G, Himori K, Sano H (2002) Tensile strength of the supraspinatus after reimplantation into a bony trough: an experimental study in rabbits. J Shoulder Elbow Surg 11:504–509
Valhmu WB, Stazzone EJ, Bachrach NM, Saed-Nejad F, Fischer SG, Mow VC, Ratcliffe A (1998) Load-controlled compression of articular cartilage induces a transient stimulation of aggrecan gene expression. Arch Biochem Biophys 353:29–36
Verstraeten AA, Mackie EJ, Hageman PC, Hilgers J, Schol DJ, De Jongh GJ, Schalkwijk J (1992) Tenascin expression in basal cell carcinoma. Br J Dermatol 127:571–574
Wertzenberger KL, Peterson HA (1980) Acquired spondylolysis and spondylolisthesis in the young child. Spine 5:437–442
Wiltse LL (1962) The etiology of spondylolisthesis. J Bone Joint Surg 44-A:539–560
Wiltse LL, Widell EH, Jackson DW (1975) Fatigue fracture: the basic lesion in isthmic spondylolisthesis. J Bone Joint Surg 57-A:17–22
Wong MW, Qin L, Lee KM, Tai KO, Chong WS, Leung KS, Chan KM (2003) Healing of bone-tendon junction in a bone trough: a goat partial patellectomy model. Clin Orthop 413:291–302
Wright IP (2003) Who was Meyerding? Spine 28:733–735
Wynne-Davies R, Scott JH (1979) Inheritance and spondylolisthesis. J Bone Joint Surg 61-B:301–305
Zippel H, Abesser EW (1965) Zur wirbelbogendysplasie und spondylolisthesis im ersten lebensdezennium. Arch Orthop Unfallchir 58:242–259
Zippel H, Runge H (1976) Pathologische anatomie und pathogenese von spondylolyse und spondylolisthesis im kindesalter. Z Orthop 114:189–201
Acknowledgements
Antibodies CIICI, 5C6, 1C6, 8A4 and 12C5 were obtained from the Developmental Studies Hybridoma Bank (DSHB) maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD., and the Department of Biology, University of Iowa, Iowa City, Iowa, under contract NO-HD-6-2915 from the NICHD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boszczyk, B.M., Boszczyk, A.A., Boos, W. et al. An immunohistochemical study of the tissue bridging adult spondylolytic defects—the presence and significance of fibrocartilaginous entheses. Eur Spine J 15, 965–971 (2006). https://doi.org/10.1007/s00586-005-0986-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-005-0986-3