Abstract
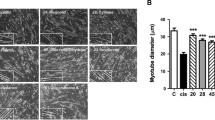
Cisplatin (CIS) is a chemotherapeutic agent known to induce cachexia. CIS causes the atrophy of skeletal muscle. Thymoquinone (TQ) is a powerful antioxidant with an anti-inflammatory effect. The aim of this study was to determine the effects of TQ on mitofusin 2 (Mfsn-2), which is one of the mitochondrial dynamics in CIS-induced muscle atrophy, and meteorin-like (MtrnL) immunoreactivity, which plays a role in energy metabolism. Twenty-eight rats were randomly divided into four groups (n = 7). While the control group was not administered, a single dose of CIS (7 mg/kg) was administered intraperitoneally (i.p) to the CIS group at the beginning of the experiment. The CIS + TQ group was administered TQ (10 mg/kg/day) oral gavage after a single dose of CIS (7 mg/kg) i.p injection at the beginning of the experiment. In the TQ group, only TQ (10 mg/kg/day) oral gavage was applied. CIS application caused atrophy in muscle tissue and increased creatine kinase (CK) and lactate dehydrogenase (LDH) levels. However, Mfsn-2, TNF, and Casp3 increased while MtrnL decreased. TQ decreased the increased biochemical parameters with CIS cognac. Increased Mfsn-2, TNF, and Casp3 levels due to CIS decreased with TQ treatment. However, the decreased MtrnL caused by CIS increased with TQ treatment. TQ may exert a protective effect in CIS-induced muscle atrophy by regulating Mfsn-2, MtrnL, TNF, and Casp3 immunoreactivities.


Similar content being viewed by others
Data availability
Data obtained and/or analyzed in the present study are available from the corresponding author upon reasonable request.
References
Bae JH, Seo DY, Lee SH, Shin C, Jamrasi P, Han J, Song W (2021) Effects of exercise on AKT/PGC1-α/FOXO3a pathway and muscle atrophy in cisplatin-administered rat skeletal muscle. Korean J Physiol Pharmacol 1;25(6):585–592. https://doi.org/10.4196/kjpp.2021.25.6.585
Bai T, Yang Y, Wu YL, Jiang S, Lee JJ, Lian LH, Nan JX (2014) Thymoquinone alleviates thioacetamide-induced hepatic fibrosis and inflammation by activating LKB1-AMPK signaling pathway in mice. Int Immunopharmacol 19(2):351–357. https://doi.org/10.1016/j.intimp.2014.02.006
Cocetta V, Ragazzi E, Montopoli M (2019) Mitochondrial involvement in cisplatin resistance. Int J Mol Sci 20:3384. https://doi.org/10.3390/ijms20143384
Conte E, Bresciani E, Rizzi L, Cappellari O, De Luca A, Torsello A, Liantonio A (2020) Cisplatin-ınduced skeletal muscle dysfunction: mechanisms and counteracting therapeutic strategies. Int J Mol Sci 13;21(4):1242. https://doi.org/10.3390/ijms21041242
Conte E, Camerino GM, Mele A, De Bellis M, Pierno S, Rana F, Fonzino A, Caloiero R, Rizzi L, Bresciani E, Ben Haj Salah K, Fehrentz JA, Martinez J, Giustino A, Mariggiò MA, Coluccia M, Tricarico D, Lograno MD, De Luca A, Torsello A, Conte D, Liantonio A (2017) Growth hormone secretagogues prevent dysregulation of skeletal muscle calcium homeostasis in a rat model of cisplatin-induced cachexia. J Cachexia Sarcopenia Muscle 8(3):386–404. https://doi.org/10.1002/jcsm.12185
Das DK, Graham ZA, Cardozo CP (2020) Myokines in skeletal muscle physiology and metabolism: recent advances and future perspectives. Acta Physiol (Oxf) 228(2):e13367. https://doi.org/10.1111/apha.13367
Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G (2011) Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 8;333(6039):233–8. https://doi.org/10.1126/science.1198973
Deger N, Ozmen R, Karabulut D (2022) Thymoquinone regulates nitric oxide synthase enzymes and receptor-interacting serine-threonine kinases in isoproterenol-induced myocardial infarcted rats. Chem-Biol Interact 365:110090. https://doi.org/10.1016/j.cbi.2022.110090
Eisele PS, Salatino S, Sobek J, Hottiger MO, Handschin C (2013) The peroxisome proliferator-activated receptor γ coactivator 1α/β (PGC-1) coactivators repress the transcriptional activity of NF-κB in skeletal muscle cells. J Biol Chem 25;288(4): 2246–60. https://doi.org/10.1074/jbc.M112.375253
Geng T, Li P, Yin X, Yan Z (2011) PGC-1α promotes nitric oxide antioxidant defenses and inhibits FOXO signaling against cardiac cachexia in mice. Am J Pathol 178(4):1738–1748. https://doi.org/10.1016/j.ajpath.2011.01.005
Geto Z, Molla MD, Challa F, Belay Y, Getahun T (2020) Mitochondrial dynamic dysfunction as a main triggering factor for ınflammation associated chronic non-communicable diseases. J Inflamm Res 14(13):97–107. https://doi.org/10.2147/JIR.S232009
Hu L, Zhou L, Wu X, Liu C, Fan Y, Li Q (2014) Hypoxic preconditioning protects cardiomyocytes against hypoxia/reoxygenation injury through AMPK/eNOS/PGC-1α signaling pathway. Int J Clin Exp Pathol 15;7(11):7378–88, PMID: 25550773
Jariyamana N, Chuveera P, Dewi A, Leelapornpisid W, Ittichaicharoen J, Chattipakorn S, Srisuwan T (2021) Effects of N-acetyl cysteine on mitochondrial ROS, mitochondrial dynamics, and inflammation on lipopolysaccharide-treated human apical papilla cells. Clin Oral Investig 25(6):3919–3928. https://doi.org/10.1007/s00784-020-03721-7
Jung TW, Lee SH, Kim HC, Bang JS, Abd El-Aty AM, Hacımüftüoğlu A, Shin YK, Jeong JH (2018) METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp Mol Med 13;50(9):1–11. https://doi.org/10.1038/s12276-018-0147-5
Kaya S, Yalçın T, Boydak M, Dönmez HH (2022) Protective effect of N-acetylcysteine against aluminum-induced kidney tissue damage in rats. Biol Trace Elem Res 1–10. https://doi.org/10.1007/s12011-022-03276-6
Kim JE, Choi HC (2010) Losartan inhibits vascular smooth muscle cell proliferation through activation of AMPactivated protein kinase. Korean J Physiol Pharmacol 14:299–304. https://doi.org/10.4196/kjpp.2010.14.5.299
Kosztelnik M, Kurucz A, Papp D, Jones E, Sigmond T, Barna J, Traka MH, Lorincz T, Szarka A, Banhegyi G, Vellai T, Korcsmaros T, Kapuy O (2019) Suppression of AMPK/aak-2 by NRF2/SKN-1 down-regulates autophagy during prolonged oxidative stress. FASEB J 33(2):2372–2387. https://doi.org/10.1096/fj.201800565RR
Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17:491–506. https://doi.org/10.1016/j.cmet.2013.03.002
Lin JF, Lin YC, Tsai TF, Chen HE, Chou KY, Hwang TI (2017) Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des Devel Ther 16(11):1517–1533. https://doi.org/10.2147/DDDT.S126464
Moreira-Pais A, Ferreira R, Gil da Costa R (2018) Platinum-induced muscle wasting in cancer chemotherapy: mechanisms and potential targets for therapeutic intervention. Life Sci 1(208):1–9. https://doi.org/10.1016/j.lfs.2018.07.010
Onesti JK, Guttridge DC (2014) Inflammation based regulation of cancer cachexia. Biomed Res Int 168407. https://doi.org/10.1155/2014/168407
Pei X, Li X, Chen H, Han Y, Fan Y (2016) Thymoquinone inhibits angiotensin II-induced proliferation and migration of vascular smooth muscle cells through the AMPK/PPARγ/PGC-1α pathway. DNA Cell Biol 35(8):426–433. https://doi.org/10.1089/dna.2016.3262
Peixoto da Silva S, Santos JM, Costa e Silva MP, Gil da Costa RM, Medeiros R (2020) Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 11(3):619–635. https://doi.org/10.1002/jcsm.12528
Powers SK, Ozdemir M, Hyatt H (2020) Redox control of proteolysis during inactivity-induced skeletal muscle atrophy. Antioxid Redox Signal 33(8):559–569. https://doi.org/10.1089/ars.2019.8000
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 5;157(6):1279–1291. https://doi.org/10.1016/j.cell.2014.03.065
Romanello V, Sandri M (2022) Implications of mitochondrial fusion and fission in skeletal muscle mass and health. Semin Cell Dev Biol 1084–9521(22):00050–00057. https://doi.org/10.1016/j.semcdb.2022.02.011
Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM (2012) A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 7;151(6):1319–31. https://doi.org/10.1016/j.cell.2012.10.050
Sakai H, Zhou Y, Miyauchi Y, Suzuki Y, Ikeno Y, Kon R, Ikarashi N, Chiba Y, Hosoe T, Kamei J (2022) Increased 20S proteasome expression and the effect of bortezomib during cisplatin-ınduced muscle atrophy. Biol Pharm Bull 45(7):910–918. https://doi.org/10.1248/bpb.b22-00177
Schakman O, Dehoux M, Bouchuari S, Delaere S, Lause P, Decroly N, Shoelson SE, Thissen JP (2012) Role of IGF-I and the TNFα/NF-κB pathway in the induction of muscle atrogenes by acute inflammation. Am J Physiol Endocrinol Metab 15;303(6):E729–39. https://doi.org/10.1152/ajpendo.00060.2012
Shahid F, Farooqui Z, Alam T, Abidi S, Parwez I, Khan F (2021) Thymoquinone supplementation ameliorates cisplatin-induced hepatic pathophysiology. Hum Exp Toxicol 40(10):1673–1684. https://doi.org/10.1177/09603271211003645
Sidharta BRA, Purwanto B, Wasita B, Widyaningsih V, Soetrisno S (2022) Single or divided administration of cisplatin can induce inflammation and oxidative stress in male Sprague-Dawley rats. Indones Biomed J 14(2):164–71. https://doi.org/10.18585/inabj.v14i2.1745
Sirago G, Conte E, Fracasso F, Cormio A, Fehrentz JA, Martinez J, Musicco C, Camerino GM, Fonzino A, Rizzi L, Torsello A, Lezza AMS, Liantonio A, Cantatore P, Pesce V (2017) Growth hormone secretagogues hexarelin and JMV2894 protect skeletal muscle from mitochondrial damages in a rat model of cisplatin-induced cachexia. Sci Rep 7(1):13017. https://doi.org/10.1038/s41598-017-13504-y
Suvarna KS, Layton C, Bancroft JD (2018) Bancroft’s theory and practice of histological techniques. 8th ed. London, UK: Elsevier health sciences 40:183
Wang S, Liu X, Lei L, Wang D, Liu Y (2022) Selenium deficiency induces apoptosis, mitochondrial dynamic imbalance, and inflammatory responses in calf liver. Biol Trace Elem Res 200(11):4678–4689. https://doi.org/10.1007/s12011-021-03059-5
Zhang J, Zheng J, Chen H, Li X, Ye C, Zhang F, Zhang Z, Yao Q, Guo Y (2022) Curcumin targeting NF-κB/ubiquitin-proteasome-system axis ameliorates muscle atrophy in triple-negative breast cancer cachexia mice. Mediators Inflamm 29:2567150. https://doi.org/10.1155/2022/2567150
Author information
Authors and Affiliations
Contributions
TY and SK took part in the study plan, designing animal experiment, data analysis, laboratory studies, and manuscript writing. All researchers read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals comply with the ethical standards of the institution or practice in which the studies are conducted. This study was carried out with the approval of Dicle University Animal Experiments Ethics Committee dated 29/03/2022 and numbered 2021/39.
Informed consent
For this type of study informed consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yalçın, T., Kaya, S. Thymoquinone may alleviate cisplatin-induced muscle atrophy in rats by regulating mitofusin 2 and meteorin-like levels. Comp Clin Pathol 32, 339–345 (2023). https://doi.org/10.1007/s00580-023-03442-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-023-03442-9