Abstract
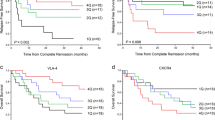
This study aimed to evaluate the bone marrow (BM) chemokine plasma levels as a biomarker for B-ALL response to induction chemotherapy and as a predictive for minimal residual disease. In the present study, we have characterized soluble BM chemokine biomarkers in 56 B-ALL patients at diagnosis and on day 15 by ELISA in parallel with minimal residual disease (MRD) after induction therapy. Our findings demonstrated that bone marrow plasma levels of CCL-2; CXCL-9; and CXCL-10 at day 0 were significantly higher as compared to their levels at day 15 (P < 0.001 for all). On the other hand, CCL-5 levels were significantly low at day 0 as compared to day 15 (P < 0.001). The soluble chemokine biomarkers at day 0 was related to MRD detection and risk severity stages. ROC curve was done in order to address the chemokine baseline level that discriminates between MRD status; the analysis revealed that the best predictive one for MRD status is CXCL-10 (predictive value 0.96 (0.81–0.999) at cutoff 63.5 pg. CXCL-10 levels at diagnosis could serve as a biomarker that could predict MRD status on day 15 post induction.

Similar content being viewed by others
Data availability
The data of the current study is available upon request to the corresponding author.
References
Aldinucci D, Colombatti A (2014) The inflammatory chemokine CCL5 and cancer progression. Mediators Inflammation 292376
Brenner AK, Nepstad I, Bruserud Ø (2017) Mesenchymal stem cells support survival and proliferation of primary human acute myeloid leukemia cells through heterogeneous molecular mechanisms. Front Immunol 8:106
Chheda ZS, Sharma RK, Jala VR, Luster AD, Haribabu B (2016) Chemoattractant receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors. J Immunol 197(5):2016–2026
Gómez AM, Martínez C, González M, Luque A, Melen G, Martínez J, Hortelano S, Lassaletta A, Madero L, Ramírez M (2015) Chemokines and relapses in childhood acute lymphoblastic leukemia: a role in migration and in resistance to antileukemic drugs. Blood Cells Mol Dis 55(3):220–227
Huffman P, Lin J, Kim S, Byrne K, Vonderheide R (2020) CCL5 mediates CD40-driven CD4+ T cell tumor infiltration and immunity. JCI Insight 5(10)
Kerr M, Magalhães-Gama F, Ibiapina H, Hanna F, Xabregas L, Alves E et al (2021) Marrow Soluble Immunological Mediators as Clinical Prognosis Biomarkers in B-Cell Acute Lymphoblastic Leukemia Patients Undergoing Induction Therapy. Front Oncol https://doi.org/10.3389/fonc.2021.696032
Khandany B, Hassanshahi G, Khorramdelazad H, Balali Z, Shamsizadeh A, Arababadi M, Ostadebrahimi H, Fatehi A, Zadeh M, Ahmadi Z, Karimabad M (2012) Evaluation of circulating concentrations of CXCL1 (Gro-α), CXCL10 (IP-10) and CXCL12 (SDF-1) in ALL patients prior and post bone marrow transplantation. Pathol Res Pract 208(10):615–619
Liu C, Yao Z, Wang J, Zhang W, Yang Y, Zhang Y et al (2020) Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ 27(6):1765–1781
Luczyński W, Stasiak-Barmuta A, Krawczuk-Rybak M, Malinowska I (2005) Assessment of selected co-stimulatory, adhesion and activatory molecules and cytokines of Th (1)/Th (2) balance in acute lymphoblastic leukemia in children. Arch Immunol Ther Exp 53(4):357–363
Meyer L, Hermiston M (2019) The bone marrow microenvironment as a mediator of chemoresistance in acute lymphoblastic leukemia. Cancer Drug Resistance 2:1164–1177
Magalhães-Gama F, Kerr M, de Araújo N, Ibiapina H, Neves J, Hanna F et al (2021) Imbalance of Chemokines and Cytokines in the Bone Marrow Microenvironment of Children with B-Cell Acute Lymphoblastic Leukemia. J Oncol | Article ID 5530650 | https://doi.org/10.1155/2021/5530650
Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K, Gajewski TF et al (2015) Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nature Communication 6:7458
Nagarsheth N, Wicha M, Zou W (2017) Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 17(9):559–572
Orleans-Lindsay JK, Barber LD, Prentice HG, Lowdell MW (2001) Acute myeloid leukaemia cells secrete a soluble factor that in hibits T and NK cell proliferation but not cytolytic function-implications for the adoptive immunotherapy of leukaemia. Clin Exp Immunol 126(3):403–411
Poeta V, Massara M, Capucetti A, Bonecchi R (2019) Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front Immunol 10:379
Stoll G, Pol J, Soumelis V, Zitvogel L, Kroemer G (2018) Impact of chemotactic factors and receptors on the cancer immune infiltrate: a bioinformatics study revealing homogeneity and heterogeneity among patient cohorts. Oncoimmunology 7(10):e1484980
Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP (2005) CXC chemokines in angiogenesis. Cytokine Growth Factor Review 16:593–609. https://doi.org/10.1016/j.cytogfr.2005.04.007
Thaiss C, Semmling V, Franken L, Wagner H, Kurts C (2011) Chemokines: a new dendritic cell signal for T cell activation. Front Immunol 2:31. https://doi.org/10.3389/fimmu.2011.00031
Tokunaga R, Zhang W, Naseem M, Puccini A, Berger M, Soni S, McSkane M, Baba H, Lenz H (2018) CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - a target for novel cancer therapy. Cancer Treat Rev 63:40–47
Yazdani Z, Baluchi I, Khandany B, Hassanshahi G (2021) Effect of Chemotherapy on CXCL1 and CXCL10 Levels in Acute Myeloid Leukemia Patients with M4/M5 Subtype. Med Lab J 15(2):5–10
Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, Stevens B, Pei S, Balys M, Ashton JM, Klemm DJ, Woolthuis CM, Stranahan AW, Park CY, Jordan CT (2016) Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell l 7;19(1):23–37
Zhu G, Yan H, Pang Y, Jian J, Achyut B, Liang X et al (2015) CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumor cell migration and promotion of host anti-tumor immunity. Oncotarget 6(41):43408–43419
Acknowledgements
The authors would like to acknowledge all the participants for sharing their time and providing consent and information necessary for the successful completion of the study.
Author information
Authors and Affiliations
Contributions
Conception: Salah Aref, Mohamed Ayed. Interpretation or analysis of data: Enas Gouda. Preparation of the manuscript: Ahmed Al Tantawy. Revision for important intellectual content: Mohamed Ayed, Enas Gouda. Supervision: Salah Aref. Ahmed Aref.
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study informed consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aref, S., Gouda, E., Al Tantawy, A. et al. Soluble bone marrow CXCL-10: a novel biomarker for B-acute lymphoblastic leukemia’s response to induction chemotherapy. Comp Clin Pathol 32, 29–35 (2023). https://doi.org/10.1007/s00580-022-03403-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03403-8