Abstract
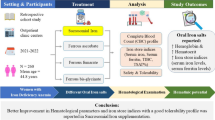
Electrolytes, particularly sodium and potassium, are highly paramount for the uptake of nutrients that are required for the proliferation, differentiation and survival of Plasmodium falciparum in the host. Sequel to this essential role, information on the interplay between electrolytes and nutrients would add to the current understanding of the malarial infection pathophysiology. To this end, we carried out a hospital-based cross-sectional study and estimated the serum levels of sodium, potassium, glucose, cholesterol and total protein in both P. falciparum-infected patients and apparently healthy patients. Our results showed that the levels of sodium, potassium, glucose, cholesterol and total protein were significantly (p < 0.05) reduced compared to their respective control groups. In addition, glucose, cholesterol and total protein had a non-significant (p > 0.05) association with the sodium and potassium, respectively, in P. falciparum-infected patients. Evidence from the present study demonstrated that P. falciparum-induced depletion in sodium and potassium seems not to play a significant role in the alterations of glucose, cholesterol and total protein during P. falciparum infection.

Similar content being viewed by others
References
Adekunle AS, Adekunle OC, Egbewale BE (2007) Serum status of selectedbiochemical parameters in malaria: an animal model. Biomed Res 18(2):109–113
Aly AS, Vaughan AM, Kappe SH (2009) Malaria parasite development in themosquito and infection of the mammalian host. Annu Rev Microbiol 63:195–221
Asahi H, Kanazawa T, Hirayama N, Kajihara Y (2005) Investigating serum factors promoting erythrocytic growth of Plasmodium falciparum. Exp Parasitol 109(1):7–15
Chukwuocha UM, Eke KN (2011) Malaria parasite status and cholesterol level ofmalaria patients in parts of the IMO River Basin of Nigeria. Asian Pac J Trop Med 4(12):993–996
Cowman AF, Crabb BS (2006) Invasion of red blood cells by malaria parasites. Cell 124(4):755–766
Frankland S, Elliott SR, Yosaatmadja F, Beeson JG, Rogerson SJ, Adisa A, Tilley L (2007) Serum lipoproteins promote efficient presentation of the malariavirulence protein PfEMP1 at the erythrocyte surface. Eukaryot Cell 6(9):1584–1594
Ginsburg H, Handeli S, Friedman S, Gorodetsky R, Krugliak M (1986) Effects of redblood cell potassium and hypertonicity on the growth of Plasmodium falciparum in culture. Z Parasitenkd 72(2):185–199
Goodyer ID, Taraschi TF (1997) Plasmodium falciparum: a simple, rapid method for detecting parasite clones in microtiter plates. Exp Parasitol 86(2):158–160
Hayakawa EH, Yamaguchi K, Mori M, Nardone G (2020) Real-time cholesterolsorting in Plasmodium falciparum-erythrocytes as revealed by 3D label-freeimaging. Sci Rep 10(1):1–12
Hilou A, Nacoulma G, Guiguemde TR (2006) In vivo antimalarial activities of extracts from Amaranthus spinosus and Boerhaavia erecta in mice. J Ethnopharmacol 103:236–240
Humeida H, Pradel G, Stich A, Krawinkel MB (2011) The effect of glucose andinsulin on in vitro proliferation of Plasmodium falciparum. J Diabetol 3:1–6
Istvan ES, Das S, Bhatnagar S, Beck JR, Owen E, Llinas M, Ganesan SM, Niles JC, Winzeler, E, Vaidya AB, Goldberg DE (2019) Plasmodium Niemann-Picktype C1-related protein is a druggable target required for parasite membrane homeostasis. Elife 8:e40529
Kirk K (2001) Membrane transport in the malaria-infected erythrocyte. Physiol Rev 81(2):495–537
Kiru AI, Bala RK, Abdulazeez AM, Bello SY, Adam AL, Suleiman SM, ShamsuA Abdulkadir ML (2018) Lipid profile and electrolyte level in malaria patients attending Muhammadu Abdullahi Wase Specialist Hospital, Kano State. Nigeria JOCAMR 5(4):1–7
Labaied M, Jayabalasingham B, Bano N, Cha SJ, Sandoval J, Guan G, Coppens I (2011) Plasmodium salvages cholesterol internalized by LDL and synthesized de novo in the liver. Cell Microbiol 13(4):569–586
Lee P, Ye Z, Van Dyke K, Kirk RG (1988) X-ray microanalysis of Plasmodiumfalciparum and infected red blood cells: effects of qinghaosu and chloroquine onpotassium, sodium, and phosphorus composition. Am J TropMed Hyg 39(2):157–165
Lowry OH (1951) Rosebrough NJ, Farr Al, and Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marshall W (2012) Total protein (serum, plasma). Ass Clin Biochem
Maitland K, Pamba A, Newton CR, Lowe B, Levin M (2004) Hypokalemia inchildren with severe falciparum malaria. Pediatr Crit Care Med 5(1):81–85
Mauritz JM, Seear R, Esposito A, Kaminski CF, Skepper JN, Warley A, Lew VL, Tiffert T (2011) X-ray microanalysis investigation of the changes in Na, K, andhemoglobin concentration in Plasmodium falciparum-infected red bloodcells. Biophys J 100(6):1438–1445
Mitamura T, Hanada K, Ko-Mitamura EP, Nishijima M, Horii T (2000) Serumfactors governing intraerythrocytic development and cell cycle progression ofPlasmodium falciparum. Parasitol Int 49(3):219–229
Moumaris M, Bretagne JM, Abuaf N (2019) Biological membranes and malaria parasites. Open Parasitol J 7(1):1–18
Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Jacobsen KH (2014) Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burdenof Disease Study 2013. Lancet 384(9947):1005–1070
Neves FA, Ventura AM, Filho MG, Libonati RM (2013) Lipid parameters in ahyperendemic area for malaria. Lipids Health Dis 12:162
Nsonwu-Anyanwu AC, Egbe ER, Osuoha UO, Inyang-Etoh PC, Offor SJ, Usoro CAO (2017) Falciparum malaria associated changes in biochemical indices inchildren. J Med Allied Sci 7(1):29–33
Orimadegun AE, Orimadegun BE (2015) Serum Apolipoprotein-A1 and CholesterolLevels in Nigerian Children with Plasmodium falciparum Infection. Med Princ Pract 24:318–324
Parida M, Thatoi PK, Choudhury A, Bhuin S, Behera S, Mohanty R (2019) Hyponatremia as a Mortality Predictor of Severe Malaria: A Hospital Based Cross-sectional Study. J Clin Diagn Res 13(2):5–8
Pillai AD, Addo R, Sharma P, Nguitragool W, Srinivasan P, Desai SA (2013) Malaria parasites tolerate a broad range of ionic environments and do not require hostcation remodelling. Mol Microbiol 88(1):20–34
Pucadyil TJ, Chattopadhyay A (2007) Cholesterol depletion induces dynamicconfinement of the G-protein coupled serotonin1A receptor in the plasma membrane ofliving cells. Biochim Biophys Acta (BBA)-Biomembranes 1768(3):655–668
Samuel BU, Mohandas N, Harrison T, McManus H, Rosse W, Reid M, Haldar K (2001) The role of cholesterol and glycosylphosphatidylinositol-anchored proteins oferythrocyte rafts in regulating raft protein content and malarial infection. J Biol Chem 276:29319–29329
Sherman IW (1979) Biochemistry of Plasmodium (malarial parasites). Microbiol Rev 43(4):453
Thanachartwet V, Krudsood S, Tangpukdee N, Phumratanaprapin W, Silachamroon U, Leowattana W, Wilairatana P, Brittenham GM, Looareesuwan S, Neild GH (2008) Hyponatraemia and hypokalaemia in adults with uncomplicated malaria in Thailand. Trop Doct 38(3):155–157
Tokumasu F, Crivat G, Ackerman H, Hwang J, Wellems TE (2014) Inwardcholesterol gradient of the membrane system in P. falciparum-infected erythrocytesinvolves a dilution effect from parasite-produced lipids. Biol Open 3(6):529–541
Usman MA, Ibrahim MA, Salman AA, Sallau AB (2020) Depletion of cholesterolcould be associated with modulation of progesterone but not other sex hormone levels during Plasmodium falciparum infection in humans: a cross-sectional study from Zaria. Nigeria Parasitol Res 119(12):4143–4150
Vial HJ, Eldin P, Tielens AG, van Hellemond JJ (2003) Phospholipids in parasitic protozoa. Mol Biochem Parasitol 126(2):143–154
Visser BJ, Wieten RW, Nagel IM, Grobusch MP (2013) Serum lipids andlipoproteins in malaria – a systematic review and meta-analysis. Malar J 12:442
White NJ (2008) Plasmodium knowlesi: The fifth human malaria parasite. Clin Infect Dis 46(2):172–173
World Health Organization (2010) World malaria report: 2010, WHO: Geneva, Switzerland. 205 p. Available at: https://apps.who.int/iris/handle/10665/44451. Retrieved 2019/04/25
World Health Organization (2019) World malaria report: 2019, WHO: Geneva, Switzerland. 232. Available at: https://www.who.int/publications/i/item/9789241565721. Retrieved 2021/08/13
Yalçin M, Sevim E, Duran A (2015) Treated with artemether-lumefantrine fiveevaluation of P. falciparum malaria cases in terms of hyponatremia andthrombocytopenia. Türkiye Parazitolojii Dergisi 39(2):155
Acknowledgements
The support of Mr. Mohammed Ibrahim of Haematology unit, Ahmadu Bello University Medical Centre, in providing necessary assistance during sample collection was highly appreciated by the authors. Furthermore, the authors wish to express their profound gratitude to the management of Ahmadu Bello University, Zaria, Nigeria, for providing the facilities used for this study.
Funding
Financial support, from any grant funding body, was not received for this study.
Author information
Authors and Affiliations
Contributions
Mukhtar Adeiza Suleiman provided conceptualisation and design. Tahiru Umaru, Karimatu Dauda, Shedrack Renan John did data acquisition. Mukhtar Adeiza Suleiman, Tahiru Umaru, Karimatu Dauda, Shedrack Renan John, Mohammed Aliyu Usman analysed and interpreted the data. Mohammed Aliyu Usman drafted the manuscript. Mukhtar Adeiza Suleiman, Tahiru Umaru, Karimatu Dauda, Shedrack Renan John, Mohammed Aliyu Usman contributed to revision, vetting and approval of the final manuscript for submission.
Corresponding author
Ethics declarations
Ethical approval
All procedures carried out in this research study were in line with the ethical standards of the institutional research committee on human experimentation and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from each individual participant in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suleiman, M.A., Umaru, T., Dauda, K. et al. Hyponatraemia and hypokalaemia relationship with alterations of glucose, cholesterol and total protein levels during human infection with Plasmodium falciparum. Comp Clin Pathol 31, 557–563 (2022). https://doi.org/10.1007/s00580-022-03354-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03354-0