Abstract
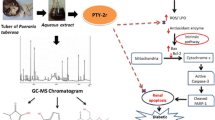
An endeavor has been made in this study to assess the effect of Phlogacanthus thyrsiflorus on hyperglycemia-induced oxidative stress along with apoptosis in liver and kidney of alloxan-administered mice. To analyze the effect of Phlogacanthus thyrsiflorus on oxidative stress in kidney and liver of diabetic mice. Furthermore, we have also examined the effect of Phlogacanthus thyrsiflorus on apoptosis in diabetic mice. Following preparation of methanolic flower extract (MFE), preliminary phytochemical screenings and acute toxicity test were carried out for MFE. Lipid peroxidation and protein carbonyl assays were determined to check the MDA level and oxidative damage in tissues of groups normal control mice (NCM), diabetic control mice (DCM), ascorbic acid–treated diabetic mice (D + AA), and MFE-treated diabetic mice (D + MFE). Histological and ultrastructural studies were conducted to evaluate any changes in tissues as well as sub-cellular organelles. The effects of MFE on caspase 3 and Bcl-2 expression in alloxan-induced diabetic mice were studied and compared against the diabetic control group of mice. Upon treatment with MFE, the diabetic mice manifested a notable depletion of malondialdehyde (MDA) and protein carbonyl levels. The ultrastructural studies divulged the capability of MFE to reinstate morphological and cellular alterations as contemplated in alloxan-induced diabetic mice. On apoptosis, the effect of MFE showed the downregulation of cysteine-dependent aspartate specific protease (caspase) 3, whereas upregulation of B-cell lymphoma-2 (Bcl-2) protein and the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labelling (TUNEL) assay illustrated a few apoptotic cells. It can be put forward that methanolic flower extract of Phlogacanthus thyrsiflorus treatment can exert hepatoprotective and nephroprotective effect via regulating hyperglycemia-induced oxidative stress and apoptosis in alloxan-administered diabetic mice.












Similar content being viewed by others
References
Bandeira S, de M da, Fonseca LJS, Vasconcelos SML (2013) Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci 14(2):3265–3284
Bora J, Sahariah P, Patar AK, Syiem D, Bhan S (2018a) Attenuation of diabetic hepatopathy in alloxan-induced diabetic mice by methanolic flower extract of Phlogacanthus thyrsiflorus Nees. J Appl Pharm 8(7):114–120
Bora J, Syiem D, Bhan S (2018b) Methanolic flower extract of phlogacanthus thyrsiflorus Nees. attenuates diabetic nephropathy in alloxan-induced diabetic mice. Asian J Pharm Clin Res 11(7):113–116
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ (2002) Hyperglycemia induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 51:1938–1948
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38
Danilova IG, Sarapultsev PA, Medvedeva SU, Getti IF, Bulavintceva TS, Sarapultsev AP (2015) Morphological restructuring of myocardium during the early phase of experimental diabetes mellitus. Anat Rec 298(2):396–407
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol 186:421–431
Francés DE, Ronco MT, Monti JA, Ingaramo PI, Pisani GB, Parody JP, Pellegrino JM, Sanz PM et al (2010) Hyperglycemia induces apoptosis in rat liver through the increase of hydroxyl radical: new insights into the insulin effect. Int J Endocrinol 205:187–200
Ghosh M (2007) Fundamentals of experimental pharmacology. Indian J Pharmacol 39(4):216
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Graham J (2002) Preparation of crude subcellular fractions by differential centrifugation. Sci World J 2:1638–1642
Harborne J (1998) Phytochemical methods : a guide to modern techniques of plant analysis, 3rd edn. Chapman and Hall, London
Hayat ME (2012) Basic techniques for transmission electron microscopy. Elsevier, Amsterdam
Inoguchi TLP, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T et al (2000) High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939–1945
Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461(7267):1071–1078
Jaiswal V (2010) Culture and ethnobotany of Jaintia tribal community of Meghalaya, North-East India- a mini review. Indian J Tradit Knowl 9(1):38–44
Kashikar VS, Tejaswita K (2011) Indigenous remedies for diabetes mellitus. Int J Pharm Pharm Sci 3(3):22–29
Kumar SS, Chaubey RC, Devasagayam TP, Priyadarsini KI, Chauhan PS (1999) Inhibition of radiation-induced DNA damage in plasmid pBR322 chlorophyllin and possible mechanism (s) of action. Mutat Res 425:71–79
Kumar SS, Devasagayam TPA, Bhushan B, Verma NC (2001) Scavenging of reactive oxygen species by chlorophyllin: an ESR study. Free Radic Res 35:563–574
Lee KS, Buck M, Houglum K, Chojkier M (1995) Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 96:2461–2468
Lenzen S (2008) The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51(2):216–226
Levine R, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S (1990) Determination of carbonyl content in oxidatively modified proteins. Meth Enzymol 186:464–478
Lipinski B (2001) Pathophysiology of oxidative stress in diabetes mellitus. J Diabetes Its Complications 15(4):203–210
Maritim AC, Sanders RA, Watkins JB (2003) Diabetes, oxidative stress, and antioxidants: a review. JBMT 17(1):24–38
Mathis D, Vence L, Benoist C (2001) Review article beta-Cell death during progression to diabetes. Nature 414:792–798
Meki ARM, Esmail EEDF, Hussein AA, Hassanein HM (2004) Caspase-3 and heat shock protein-70 in rat liver treated with aflatoxin B1: effect of melatonin. Toxicon 43:93–100
Moreli JB, Santos JH, Calderon IMP (2014) DNA damage and its cellular response in mother and fetus exposed to hyperglycemic environment. Biomed Res Int 676758
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pan HZ, Zhang L, Guo MY et al (2010) The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol 47:71–76
Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, Dianzani MU (1992) Vitamin E dietary supplementation protects against carbon tetrachlorideinduced chronic liver damage and cirrhosis. Hepatology 16:1014–1021
Patar AK, Bhan S, Syiem D, Sharma A (2017) Ameliorative effect of chlorophyllin on oxidative stress in experimental model of diabetes. Int J Phytomed 8:506–513
Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci 4(2):89–96
Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C (2013) Oxidative stress in diabetes: implications for vascular and other complications. Int J Mol Sci 14(11):21525–21550
Pizzino G, Irrera N, Cucinotta et al (2017) Oxidativestress:harmsandbenefitsfor human health. Oxid Med Cell Longev 8416763
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2(2):219–236
Rashid K, Bhattacharya S, Sil PC (2012) Protective role of Dsaccharic acid- 1, 4-lactone in alloxan induced oxidative stress in the spleen tissue of diabetic rats is mediated by suppressing mitochondria dependent apoptotic pathway. Free Radic Res 46:240–252
Saio V, Syiem D, Sharma R (2012) Effect of Potentilla fulgens on lipid peroxidation and antioxidant status in alloxan-induced diabetic mice. J Basic Clin Pharm 3:249–254
Sharma D, Kumar SS, Sainis KB (2007) Antiapoptotic and immunomodulatory effects of chlorophyllin. Mol Immunol 44:347–359
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87(1):4–14
Sindhu RK, Koo JR, Roberts CK, Vaziri ND (2004) Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin Exp Hypertens 26:43–53
Syiem D, Gareth S, Khup PZ, Khongwir BS, Kharbuli B, Kayang H (2002) Hypoglycemic effect of Potentilla fulgens L. in normal and alloxan-induced diabetic mice. J Ethnopharmacol 83:55–61
Thiyagarajan P, Murugan RS, Kavitha K, Anitha P, Prathiba D, Nagini S (2012) Dietary chlorophyllin inhibits the canonical NF-κB signaling pathway and induces intrinsic apoptosis in a hamster model of oral oncogenesis. Food Chem Toxicol 50:867–876
Wani SA, Shah KW, Ahmad MA (2012) Preliminary phytochemical investigation and thin layer chromatography of Rheum emodi. Int Res J Pharm 3(4):176–177
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care 27(5):1047–53
Wright E, Scism-Bacon JL, Glass LC (2006) Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract 60(3):308–314
Yang XH, Sladek TL, Liu X, Butler BR, Froelich CJ, Thor AD (2001) Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin and etoposide-induced apoptosis. Cancer Res 61:348–354
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bora, J., Sahariah, P., Dey, A. et al. Phlogacanthus thyrsiflorus Nees. modulates hepatic and renal apoptosis via attenuation of oxidative stress in alloxan-administered mice. Comp Clin Pathol 31, 483–495 (2022). https://doi.org/10.1007/s00580-022-03347-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03347-z