Abstract
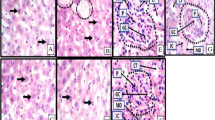
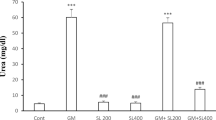
This study evaluated the effects of methanolic leaf extract of Pterocarpus santalinoides (MLEPS) on the recovery from gentamicin-induced nephrotoxicity in albino rats. Fresh leaves of P. santalinoides were collected, dried, pulverized, and extracted by cold maceration technique using 80% methanol. Thirty male albino rats randomly assigned into six groups (A–F) of five rats each were used for the study. Nephrotoxicity was induced in groups A–E by intra-peritoneal administration of gentamicin (GM) once a day at 100 mg/kg for 10 days. Thereafter, the nephropathic (NP) rat groups (A–E) were used to assess the therapy with MLEPS. Group A was given drinking water (negative control). Groups B, C, and D were treated orally with MLEPS at 125, 250, and 500 mg/kg, respectively. Group E was treated orally with silymarin at 200 mg/kg (positive control). Group F was not treated with GM and MLEPS (normal control). Parameters measured relating to kidney function were serum creatinine and urea concentrations, urine protein level, and 24-h water consumption. All the parameters were significantly (p < 0.05) increased on day 10 post-treatment with GM, which corresponds with day 0 post-treatment with MLEPS. Serum creatinine levels of the rat group treated with 500 mg/kg MLEPS were significantly (p < 0.05) lower than that of the untreated control on days 5 and 10 post-treatment with extract (PTWE). The serum urea levels of the rat group treated with 500 mg/kg MLEPS were comparable (p > 0.05) to that of the group F (normal control) rats on days 5 and 10 PTWE, though it was not significantly (p > 0.05) different from the untreated control (group A). Urine protein levels of rats treated with 500 mg/kg MLEPS (group D) were lower and comparable to that of rats treated with silymarin (group E). Also, treatment with MLEPS at 250 and 500 mg/kg led to amelioration of renal tubular necrosis in the NP rats. It was concluded that treatment with MLEPS at 500 mg/kg significantly improved recovery time from GM-induced kidney damage in rats, based on the enhancement of creatinine and urea clearance from blood, reduction of protein loss in urine, and amelioration of histological lesions.


Similar content being viewed by others
References
Abdelmeguid NE, Chmaisse HN, Abouzeinab NS (2010) Protective effect of silymarin on cisplatin-induced nephrotoxicity in rats. Pakistan J Nutr 9(7):624–636
Abdel-Naim AB, Abdel-Wahab MH, Attia FF (1999) Protective effects of vitamin E and probucol against gentamicin-induced nephrotoxicity in rats. Pharmacol Res 40(2):183–187
Abouzeinab NS (2015) Antioxidant effect of silymarin on cisplatin-induced renal oxidative stress in rats. J Pharmacol Toxicol 10(1):1–19
Adesina SK (1982) Studies on the Nigeria herbal medicinal plants. Int J Crude Drug Res 20(2):93–100
Adetunji JA (2007) Reviewing Pterocarpus species and their distribution. Afr J Tradit Complement Altern Med 4(2):23–36
Aja PM, Ezeudeh NL, Umahi BG, Ugwu-Okechukwu PC, Enechi OC, Nweke OL, Ogbu-Patience N (2016) Hepatoprotective effect of ethanol extract of Pterocarpus santalinoides leaf on carbon tetrachloride induced albino rats. Carib J Sci Tech 4:882–895
Akaniro-Ejim EN, Ibe MI, Engwa GA (2018) Polyphenol content and in vitro anti-oxidant activity of aqueous-ethanol extract of Pterocarpus soyauxii and Pterocarpus santalinoides. Glob J Med Res 18(2):1–8
Ali BH (1995) Gentamicin nephrotoxicity in humans and animals: some recent research. Gen Pharmacol 26:1477–1487
Ali BH, Za’abi MA, Blunden G, Nemmar A (2011) Experimental gentamicin nephrotoxicity and agents that modify it: a mini-review of recent research. Basic Clin Pharmacol Toxicol 109:225–232
Amiri M, Motamedi P, Vakili L, Dehghani N, Kiani F, Taheri Z, Torkamaneh S, Nasri P, Nasri H (2014) Beyond the liver protective efficacy of silymarin; bright renoprotective effect on diabetic kidney disease. J Nephropharmacol 3(2):25–26
Anowi CF, Okonkwo C, Agbata CA, Ezeokafor E (2012) Preliminary phytochemical screening, evaluation of acute toxicity and antipyretic activity of methanolic extract of Pterocarpus santalinoides (Fabaceae). Int J Pharm Phytopharm Res 1(6):343–346
Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A (2005) Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicol 212(2–3):116–123
AVMA (2013) Guidelines for the euthanasia of animals: 2013 edition. American Veterinary Medical Association (AVMA), Illinois, USA, pp 48-50
Azhar-Alarm MM, Javed K, Jafri MA (2005) Effects of Rheum emodi (Revand Hindi) on renal functions in rats. J Ethnopharmacol 96:121–125
Blass KG, Thiebert RJ, Lam LK (1974) A study of the mechanism of the Jaffe reaction. J Clin Chem Clin Biochem 12:336–343
Bolliger AP, Everds NE (2010) Hematology of laboratory rodents: mouse (Mus musculus) and rat (Rattus norvegicus). In: Weiss DJ, Wardrop KJ (eds) Schalm’s veterinary hematology, 6th edn. Wiley-Blackwell, Iowa, pp 852–862
Bothon FTD, Moustapha M, Bogninou GS, Dossa PA, Yehouenou B, Medoatinsa SE, Noudogbessi JP, Avlessi F, Sohounhloue DCK (2014) Chemical characterization and biological activities of Newbouldia laevis and Pterocarpus santalinoides leaves. Bull Environ Pharmacol Life Sci 3(11):9–15
Butt WI, Bhatti M, Akhter N, Tahir M (2017) Effects of silymarin on gentamicin-induced nephrotoxicity in albino rats. Pak J Med Health Sci 11(2):735–738
Du XH, Yang CL (1994) Mechanism of gentamicin nephrotoxicity in rats and the protective effect of zinc-induced metallothionein synthesis. Nephrol Dial Transplant 9:135–140
Eisenberg JM, Koffer H, Glick HA, Connell ML, Loss LE, Talbot GH, Schusterman NH, Strom BL (1987) What is the cost of nephrotoxicity associated with aminoglycosides? Ann Intern Med 107(6):900–909
Enemali MO, Udedi SC, Ubaoji KI, Haruna GS, Augustine E (2019) Modulatory effect of leaf extracts of Pterocarpus santalinoides hook F. (red sandal wood) on acetamoniphen-induced hepatotoxicity in albino rats. FUW Trnds Sci Technol J 4(2):366–371
Eshraghi-Jazi F, Talebi A, Mirsaeedi FS, Ahmadian S, Moslemi F, Nematbakhsh M (2016) Gentamicin-induced nephrotoxicity: the role of sex hormones in gonadectomized male and female rats. Scientifica (Cairo): article ID 5025097, 9 pages https://doi.org/10.1155/2016/5025097
Eze SO, Cornelius C, Okereke HC (2012) Phytochemical and antimicrobial analysis of the stem bark of Pterocarpus santalinoides (Nturu ukpa). Asian J Nat Appl Sci 1(3):26–32
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Path 13:156–159
Gordon JA, Dillingham MA, Guggenheim SJ, Grossfeld PD, Anderson RJ (1983) The renal concentrating defect after gentamicin administration in the rat. J Lab Clin Med 101(6):903–910
Houghton DC, Hartnett M, Campbell-Boswell M, Porter G, Bennett W (1976) A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am J Pathol 82(3):589–612
Igoli JO, Ogaji OG, Tor-Anyiin TA, Igoli NP (2005) Traditional medicine practice amongst the Igede people of Nigeria, part II. Afr J Trad Complement Altern Med 2(2):134–152
Ihedioha TE, Asuzu IU, Anaga AO, Ihedioha JI (2019) Hepatoprotective and antioxidant activities of Pterocarpus santalinoides methanol leaf extract. Afri J Pharm Pharmacol 13(18):359–373
Ihedioha TE, Okechukwu VN, Ihedioha JI (2018) Effects of aqueous leaf infusion of Pterocarpus santalinoides DC on serum lipid profile of Guinea pigs (Carvia porcellus). J Complement Med Res 7(2):154–160
Ihedioha TE, Onwuegbuka LU, Ihedioha JI (2017) Hepatoprotective effects of methanol leaf extract of Pterocarpus santalinoides DC on acetaminophen-induced liver damage in albino rats (Rattus norvegicus). Nig J Anim Prod 45(5):136–145
Kaloyanides GJ, Munoz-Pastoriza E (1980) Aminoglycoside nephrotoxicity. Kidney Int 18:571–582
Karahan I, Atessahin A, Yilmaz S, Ceribasi AO, Sakin F (2005) Protective effects of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicol 215(3):198–204
Kariagina I, Slepysheva VV, Koslov AV (1996) A rapid method for the quantitative determination of protein in urine. Klinicheskaia Diagnostica 6:28–37
Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M (2011) Silymarin; a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci 14(4):308–317
Keay RWJ (1989) Trees of Nigeria. In: Keay RWJ, Onochie CFA, Stanfied DP (eds) A revised version of Nigeria trees (1960, 1964), 1st edn. Claredon press, Oxford, United Kingdom, p 476
Khan I, Khan M, Saleem M (2008) Pathological and biochemical effects of intramuscular gentamicin administration in chickens. Turkish J Vet Anim Sci 32(5):345–351
Krause KM, Serio AW, Kane TR, Connolly LE (2016) Aminoglycosides: an overview. Cold Spring Harb Perspect Med 6(6):a027029
Lakshmi BVS, Sudhakar M (2010) Protective effect of Zingiber officinale on gentamicin-induced nephrotoxicity in rats. Int J Pharmacol 6:58–62
Lamb EJ, Price CP (2008) Creatinine, urea and uric acid. In: Burtis CA, Ashwood ER, Bruns DE (eds) Tietz fundamentals of clinical chemistry, 6th edn. Saunders Elsevier, Missouri, pp 363–372
Lawal IO, Uzokwe NE, Igbanugo ABI, Adio AF, Awosan EA, Nwogwugwu JO, Faloye B, Olatunji BP, Adesoga AA (2010) Ethno medicinal information on collation and identification of some medicinal plants in research Institutes of South-West Nigeria. Afr J Pharm Pharmacol 4(1):001–007
Moghaddam AH, Javaheri M, Nabavi SF, Mahdavi MR, Nabavi SM, Ebrahimzadeh MA (2010) Protective role of Pleurotus porrigans (Angel’s wings) against gentamicin-induced nephrotoxicity in mice. Eur Rev Med Pharmacol Sci 14:1011–1014
Morin JP, Viotte G, Vandewalle A, Van Hoof F, Tulkens P, Fillastre JP (1980) Gentamicin-induced nephrotoxicity: a cell biology approach. Kidney Int 18:583–590
Nakajima T, Hishida A, Kato A (1994) Mechanisms for protective effects of radical scavengers on gentamicin-mediated nephropathy in rats. Am J Phys 266:F425–F431
Nowacek JM (2010) Fixation and tissue processing. In: Kumar GL, Kierman JA (eds) Dako pathology education guide: special stains and H & E, 2nd edn. Dako, California, North America, pp 141–152
Odeh IC, Tor-Anyiin A (2014) Phytochemical and antimicrobial evaluation of leaf-extracts of Pterocarpus santalinoides. Eur J Med Plants 4(1):105–111
Offor CE, Nwankwegu NJ, Ugwu-Okechukwu PC, Aja PM (2015) The effects of ethanol leaf extract of Pterocarpus santalinoides on liver enzymes of albino rats. American-Eurasian J Agric Environ Sci 15(5):920–922
Ogan MT (2004) Trees of Nigeria. J Complement Integr Med 123(1):125–129
Ogundipe DJ, Akomolafe RO, Sanusi AA, Imafidon CE, Olukiran OS, Oladele AA (2016) Effects of two weeks administration of Ocimum gratissimum leaf on feeding pattern and markers of renal function in rats treated with gentamicin. Egyptian J Basic Appl Sci 3(3):219–231. https://doi.org/10.1016/j.ejbas.2016.07.002
Okpo S, Ching F, Nwankwo E (2009) Evaluation of the anti-inflammatory effects of the aqueous leaf extract of Pterocarpus santalinoides. West Afr J Pharmacol Drug Res 25(1):23–26
Okwu DE (2005) Phytochemical, vitamin and mineral contents of two Nigerian medicinal plants. Int J Mol Med Adv Sci 1(4):375–381
Okwuosa CN, Unekwe PC, Achukwu PU, Udeani TKC, Ogidi UH (2011) Glucose and triglyceride lowering activity of Pterocarpus santalinoides leaf extracts against dexamethasone induced hyperlipidemia and insulin resistance in rats. Afr J Biotech 10(46):9415–9420
Pedrazza-Chaverri J, Gonzales-Orozco AE, Maldonado PD, Barrera D, Medina-Campos ON, Hernandez-Pando R (2003) Dialyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur J Pharmacol 473(1):71–78
Pietta PG (2000) Flavonoids as anti-oxidants. J Nat Prod 63:1035–1042
Pradhan SC, Girish C (2006) Hepatoprotective herbal drug silymarin, from experimental pharmacology to clinical medicine. Indian J Med Res 124:491–504
Rafieian-Kopaie M, Nasri H (2012) Silymarin and diabetic nephropathy. J Renal Injury Prev 1(1):3–5
Randjelovic P, Veljkovic S, Stojiljkovic N, Jankovic-Velickovic L, Sokolovic D, Stoiljkovic M, Ilic I (2012a) Salicylic acid attenuates gentamicin-induced nephrotoxicity in rats. Scientific World J 2012:390613
Randjelovic P, Veljkovic S, Stojiljkovic N, Sokolovic D, Ilic I (2017) Gentamicin nephrotoxicity in animals: current knowledge and future perspectives. EXCLI J 16:388–399
Randjelovic P, Veljkovic S, Stojiljkovic N, Velickovic L, Sokolovic D, Stoiljkovic M, Ilic I (2012b) Protective effect of selenium on gentamicin-induced oxidative stress and nephrotoxicity in rats. Drug Chem Toxicol 35:141–148
Saller R, Melzer J, Reichling J, Brignoli R, Meier R (2007) An updated systematic review of the pharmacology of silymarin. Res Complem Med 14(2):70–80
Shahbazi F, Dashti-Khavidaki S, Khalili H, Lessan-Pzeshki M (2012) Potential renoprotective effects of silymarin against nephrotoxic drugs: a review of literature. J Pharm Pharma Sci 15(1):112–123
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exptl Physiol 82:291–295
Surai PF (2015) Silymarin as a natural anti-oxidant: an overview of the current evidence and perspectives. Antioxidants 4:240–247
Vakulenko SB, Mobashery S (2003) Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev 16(3):430–450
Valko M, Liebrfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME (2007) Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity in dogs. J Vet Pharmacol Therap 30(5):477–481
Winsor L (1994) Tissue processing. In: Woods A, Ellis R (eds.) Laboratory histopathology. Churchill Livingstone, New York, pp 4.2-1 – 4.2-39
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ihedioha, T.E., Nnadozie, A.E., Asuzu, I.U. et al. Effects of methanolic leaf extract of Pterocarpus santalinoides on the recovery from gentamicin-induced nephrotoxicity in albino rats. Comp Clin Pathol 29, 1209–1217 (2020). https://doi.org/10.1007/s00580-020-03173-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-020-03173-1