Abstract
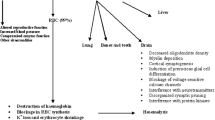
Lead (Pb) poisoning has been recognised as a clinical condition since ancient times. The anaemia frequently observed in Pb poisoning is thought to result from the shortening of erythrocytes, life span and inhibition of key enzymes of haem synthesis [viz. delta aminolevulinic acid dehydratase (ALAD), pyrimidine 5′-nucleotidase type 1 (P5N-1) and ferrochelatase]. However, the exact mechanism by which Pb shortens erythrocyte life span is still debatable. In the present study, the effect of ingested Pb on erythrocyte membrane sialic acid content and osmotic fragility of New Zealand White (NZW) rabbits was studied. Indices of Pb exposure, RBC counts, haemoglobin concentration (Hb), haematocrit (PCV), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) of rabbits were investigated. Exposure to Pb significantly decreased (p < 0.05) RBC counts, Hb levels, MCV and MCH. Similarly, exposure to Pb significantly decreased erythrocyte sialic acid content and increased the erythrocyte osmotic fragility of Pb-treated animals. It is speculated that decreases in membrane sialic acid content due to subchronic low-level Pb exposure shortens red cell life span as observed by increases in osmotic fragility.


Similar content being viewed by others
Change history
06 June 2018
The original version of this article contained a mistake. The BLLS value 53.92±14.06 shown in Table 1, Row 7, Column 8 should read 24.67±2.30.
References
Adamu S, Useh NM, Ibrahim ND, Nok AJ, Esievo KAN (2009) Erythrocyte surface sialic acids depletion as predisposing factor to erythrocyte destruction in sheep experimental model of African trypanosomosis: a preliminary report. Slov Vet Res 46(1):19–28
Ahyayauch H, Sansar W, Rendón-Ramírez A, Goñi FM, Bennouna M, Gamrani H (2013) Effects of chronic and acute lead treatments on the biophysical properties of erythrocyte membranes, and a comparison with model membranes. FEBS Open Bio 3:212–217
Albahary C (1972) Lead and haemopoiesis. Am J Med 52:367–378
Aminoff D (1961) Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J 81:384–392
Baker FJ, Silverton RE, Pallister CJ (2001) Baker and Silverton’s introductory to medical laboratory technology (Nigerian edition). Bounty Press, Nigeria, pp 380–381
Bitto E, Bingman CA, Wesenberg GE, McCoy JG, Phillips GN Jr (2006) Structure of pyrimidine 5′-nucleotidase type 1. Insight into mechanism of action and inhibition during lead poisoning. J Biol Chem 281(29):20521–20529
Bulai T, Bratosin D, Artenie V, Montreuil J (2003) Uptake of sialic acid by human erythrocyte. Characterizatioin of a transport system. Biochemie 85:241–244
Buratai LB, Nok AJ, Ibrahim S, Umar IA, Esievo KAN (2006) Characterization of sialidase from bloodstream froms of Trypanosma vivax. Cell Biochem Funct 24:71–77
CDC (Centers for Disease Control and Prevention) (2013) Blood lead levels in children aged 1–5 years, United States 1999-2010. MMWR, 46(7): 141–146
Chang YC, Uchiyama S, Varki A, Nizet V (2012) Leukocyte inflammatory responses provoked by pneumococcal sialidase. MBio 3(1):e00220–e00211
Cheesbrough M (2005) District laboratory practice in tropical countries, 2nd edition (part 2). Cambridge University Press, Cambridge, UK. pp 295–299
Chen GY, Chen X, King S, Cavassani KA, Cheng J, Zheng X, Cao H, Qu J, Fang D, Wu W, Bai XF, Liu JQ, Woodiga SA, Chen C, Sun L, Hogaboam CM, Kunkel SL, Zheng P, Liu Y (2012) Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol 29(5):1–8
CIOMS/ILAS (Council for International Organisation of Medical Sciences and International Council for Laboratory Animal Sciences) (2012). International guiding principle for biomedical research involving animals [pdf]. Available at <https://grants.nih.gov/grants/olaw/guiding_principles_2012.pdf> [Accessed 9 March 2017]
Coles EH (1986) Veterinary clinical pathology, 2nd edn. W. B. Saunders, Philadelphia US, pp 192–224
Coustou V, Plazolles N, Guegan F, Baltz T (2012) Sialidases play a key role in infection and anaemia in Trypanosoma congolense animal trypanosomiasis. Cellular Microbiol 14(3):431–445
Dodge JT, Mitchell C, Hanahan DJ (1963) The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys 100:119–130
Donaldson WE, Knowles SO (1993) Is lead toxicosis a reflection of altered fatty acid composition of membranes. Comp Biochem Physiol 104:377–379
Garg SK (2007) Veterinary toxicology. S. K. Jain CBS Publishers and Distributors, New Delhi, pp 24–29
Ghazaei C, Ahmadi M, Jazani NH (2010) Detection of neuraminidase activity in Pseudomonas aeruginosa PAO1. Iranian J Basic Med Sci 13(3):69–75
Griggs RC (1964) Lead poisoning: hematologic aspects. Prog Hematol 4:117–137
Heuermann D, Roggentin P, Kleineidam RG, Schauer R (1991) Purification and characterization of a sialidase from Clostridium chauvoei NC08596. Glycoconj J 8:95–101
Jang WH, Lin KM, Kim K, Noh JY, Kang S, Chang YK, Chung JH (2011) Low level of lead can induce phosphatidylserine exposure and erythrophagocytosis: a new mechanism underlying lead-associated anaemia. Toxicol Sci 122:177–184
Karai I, Fukumoto K, Kageyama K, Horiguchi S (1982) Effect of lead in vitro on water metabolism and osmotic fragility of human erythrocytes. Br J Ind Med 39(3):295–299
Kempe DS, Lang PA, Eisele K, Klarl BA, Weider T, Huber SM, Duranton C, Lang F (2005) Stimulation of erythrocytes phosphatidylserine exposure by lead ions. Am J Physiol Cell Physiol 288:C396–C402
Kahn CM (ed) (2010) The Merck Veterinary Manual. 10th Edn. Merck & Co., Inc. Kenilworth, p 2824
Kim Y, Yoo CI, Lee CR, Lee JH, Lee H, Kim SR, Chang SH, Lee WJ, Hwang CH, Lee YH (2002) Evaluation of activity of erythrocytes pyrimidine 5′-nucleotidase (P5N) in lead exposed workers: with focus on the effect on haemoglobin. Ind Health 40:23–27
Knowles SO, Donaldson WE (1997) Lead disrupts eicosanoid metabolism, macrophage function and diseases resistant in birds. Biol Trace Elem Res 60:13–26
Maiti AK, Saha NC, Paul G (2010) Effect of lead on oxidative stress, Na+K+atpase activity and mitochondrial electron transport chain activity of the brain of Clarias batrachus L. Bull Environ Contam Toxicol 84:672–676
Mally M, Shin H, Paroz C, Landmann R, Cornelis GR (2008) Capnocytophaga canimorsus: a human pathogen feeding at the surface of epithelial cells and phagocytes. PLoS Pathog 4:e1000164
Mrugesh T, Dipa L, Manishika G (2011) Effect of lead on human erythrocytes: an in vitro study. Acta Pol Pharm Drug Res 68(5):653–656
Mulligan C, Geertsma ER, Severi E, Kelly DJ, Poolman B, Thomas GH (2009) The substrate-binding protein imposes directionality on an electrochemical sodium gradient-driven TRAP transporter. Proc Natl Acad Sci U S A 106:1778–1783
Nguyen DB, Wagner-Britz L, Maia S, Steffen P, Wagner C, Kaestner L, Bernhardt I (2011) Regulation of phosphatidylserine exposure in red blood cells. Cell Physiol Biochem 28(5):847–856
Nok AJ, Nzeribe HC, Yako SK (2003) Trypanosoma evansi: surface localization, properties and hydrolysis of ghost red blood cells and brain cells-implications in trypanosomiasis. Z Naturforsch 58c:594–601
Parpart AK, Lorenz PB, Parpart ER, Gregg JR, Chase AM (1947) The osmotic resistance (fragilità) of human red cells. J Clin Invest 26:636–640
Patrick L (2006) Lead toxicity, a review of the literature. Part 1: exposure, evaluation and treatment. Altern Med Rev 11:2–22
PEMM (Perkin-Elmer Inc. Methods Manual) (1996) Analytical methods for atomic absorption spectroscopy. Perkin-Elmer Corp., Norwalk, p 47 132–299
Schalm OW, Jain NC, Caroll EJ (1975) Textbook of veterinary haematology, 2nd edn. Published by Lea and Fabiger, Philadelphia, pp 129–250
Schauer R, Kamerling JP (1997) Chemistry, biochemistry and biology of sialic acids. In: Glycoproteins II (ed) Montreuil J, Vliegenthart JFG, Schachter H. Elsevier, Amsterdam, pp 241–400
Schwerdtfeger SM, Melzig MF (2010) Sialidase in biological systems. Pharmazie 65:551–561
Severi E, Randle G, Kivlin P, Whitfield K, Young R, Moxon R, Kelly D, Hood D, Thomas GH (2005) Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol Microbiol 58:1173–1185
Shinozuka T (1994) Changes in human red blood cells during aging in vivo. Keio J Med 43:155–163
Suradkar SG, Ghodasara DJ, Vohol P, Patel J, Jaiswal V, Prajapati KS (2009) Haemato-biochemical alterations induced by lead acetate toxicity in Wistar rats. Vet World 2(11):429–431
Tanner MJ (1993) The major integral proteins of the human red cell. Baillieres Clin Haematol 6:333–356
Terayama K (1993) Effects of lead on electrophoretic mobility, membrane sialic acid, deformability and survival of rat erythrocytes. Ind Health 13(3):113–126
Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev 49(3):529–554
Useh NM (2002) The production and characterization of neuraminidase (sialidase) from Clostridium chauvoei (Jakari strain). M.Sc. Ahmadu Bello University, Zaria
Useh NM, Ajanusi JO, Esievo KAN, Nok AJ (2006) Characterization of Sialidase (neuraminidase) isolated from Clostridium Chauvoei (Jakari strain). Cell Biochem Funct 24(4):347–352
Useh NM, Arimie DI, Balogun EO, Ibrahim NDG, Nok AJ, Esievo KAN (2012) Desialylation modulates alkaline phosphatase activity in Zebu cattle experiemntaly infected with Clostridium chauvoei: a novel report. Jordan J Biol Sci 5(4):265–268
Valentine WN, Paglia DE, Fink K, Maldokoro G (1976) Lead poisoning: association with haemolytic anaemia, basophilic stippling, erythrocytes pyrimidine 5′- nucleotidase deficiency and intraerythrocytic accumulation of pyrimidine. J Clin Invest 58:926–932
Varki A (2007) Glycan-based interaction involving vertebrate sialic acid-recognizing proteins. Nature 446:1023–1029
Varki A (2011) Evolutionary forces shaping the golgi glycosylatioin machinery: why cell suface glycans are universal to living cells. Cold Spring Harb Perspect Biol 3:a005462
Varki A, Schauer R (2009) Sialic acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertoxxi CR, Hart GW, Etzler MM (eds) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, New York, pp 199–218
von Itzstein M (2007) The war against influenza: discovery and development of sialidase inhibitors. Nat Rev Drug Discov 6:967–974
Walski T, Chludzińska L, Komorowska M, Witkiewicz W (2014) Individual osmotic fragility distribution: a new parameter for determination of the osmotic properties of human red blood cells. BioMed Res Intern 2014(162102):1–6
Warren L (1994) Bound carbohydrates in nature. Cambridge University Press, Cambridge
Wesseling MC, Wagner-Britz L, Huppert H, Hanf B, Hertz L, Nguyen DB, Bernhardt I (2016a) Phosphatidylserine exposure in human red blood cells depending on cell age. Cell Physiol Biochem 38:1376–1390
Wesseling MC, Wagner-Britz L, Nguyen DB, Asanidze S, Mutua J, Mohamed N, Hanf B, Ghashghaeinia M, Kaestner L, Bernhardt I (2016b) Novel insights in the regulation of phosphatidylserine exposure in human red blood cells. Cell Physiol Biochem 39:1941–1954
Yangho KIM, Yoo C, Lee CR, Lee JH, Lee H, Kim S, Chang S, Lee W, Hwang C, Lee YH (2002) Evaluation of activity of erythrocytes pyrimidine 5′-nucleotidase (P5N) in lead exposed workers: with focus on the effect on haemoglobin. Ind Health 40:23–27
Acknowledgements
Assistance from Prof Nickodemus M. Useh of the Department of Veterinary Pathology and Microbiology, College of Veterinary Medicine, Federal University of Agriculture, Makurdi, in design, methodology evaluation and review is hereby acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahur, V.M., Anika, S.M. & Udem, S.C. Perturbations of erythrocyte membrane integrity by subchronic low-level lead exposure in New Zealand white rabbits. Comp Clin Pathol 27, 893–900 (2018). https://doi.org/10.1007/s00580-018-2679-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2679-4