Abstract
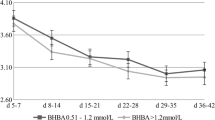
The aim of the present study was to evaluate the relationship between bovine metabolic parameters and severity of clinical endometritis and the likelihood of recovery from endometritis following PGF2α therapy. Clinical endometritis was diagnosed by ultrasonographic examination from 26 to 32 days postpartum (day 0 of experiment) and on presence of uterine discharge. The severity of endometritis was classified on a 3-point scale. Those cows with a corpus luteum (n = 113), received PGF2α (LUTALYSE, Pfizer, Australia) on day 0. Serum non-esterified fatty acids (NEFA), β-hydroxybutyric acid (BHBA), urea, and direct and total bilirubin concentrations of endometritis cows were measured on days 0, 7, and 14 of the experiment. Clinical recovery was evaluated on day 14. Recovered (RC) cows had lower NEFA values than unrecovered (URC) ones irrespective of the grade at days 7 and 14 (P < 0.02). The highest NEFA levels for all grades of the RC cows were observed on day 0. After treatment, the NEFA values of the RC cows were lower for all grades at days 7 (27.10 ± 2.41, 27.31 ± 0.81 and 22.32 ± 2.08 mg/dL, respectively; P = 0.01) and 14 (14.47 ± 1.99, 10.52 ± 0.22, and 15.32 ± 1.39 mg/dL, respectively; P < 0.02). Amounts of NEFA in URC cows increased from day 0 to day 7 for all grades of endometritis (P < 0.02). Other metabolites did not show significant relation (P > 0.05). NEFA can be considered a key metabolite for following the response to PGF2α therapy of cows having clinical endometritis.
Similar content being viewed by others
References
Borsberry S, Dobson H (1989) Periparturient diseases and their effect on reproductive performance in five dairy herds. The Vet Rec 124:217–219
Butler WR, Everett RW, Coppock CE (1981) The relationships between energy balance, milk production, and ovulation in postpartum Holstein cows. J Anim Sci 53:742–748
Chapinal N, Carson ME, LeBlanc SJ, Leslie KE, Godden S, Capel M, Santos JEP, Overton MW, Duffield TF (2012) The association of serum metabolites in the transition period with milk production and early-lactation reproductive performance. J Dairy Sci 95(3):1301–1309
Doepel L, Lapierre H, Kennelly JJ (2002) Peripartum performance and metabolism of dairy cows in response to prepartum energy and protein intake. J Dairy Sci 85:2315–2334
Drackley JK, Overton TR, Douglas NG (2001) Adaptations of glucose and long chain fatty acid metabolism in liver of dairy cows during the periparturient period. J Dairy Sci 84:100–112
Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ (2010) Risk factors for postpartum uterine diseases in dairy cows. J Dairy Sci 93:5764–5771
Duffield TF, Lissemore KD, McBride BW, Leslie KE (2009) Impact of hyperketonemia in early lactation dairy cows on health and production. J Dairy Sci 92:571–580
Fourichon C, Seegers H, Malher X (2000) Effect of disease on reproduction in the dairy cow: a meta-analysis. Theriogenology 53:1729–1759
Galvão KN, Flaminio MJBF, Brittin SB, Sper R, Fraga M, Caixeta L, Ricci A, Guard CL, Butler WR, Gilbert RO (2010) Association between uterine disease and indicators of neutrophil and systemic energy status in lactating Holstein cows. J Dairy Sci 93:2926–2937
Gautam G, Nakao T, Koike K, Long ST, Yusuf M, Ranasinghe RMSBK, Hayashi A (2010) Spontaneous recovery or persistence of postpartum endometritis and risk factors for its persistence in Holstein cows. Theriogenology 73:168–179
Gilbert RO, Shin ST, Guard CL, Erb HN, Frajblat M (2005) Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 64:1879–1888
Giuliodori MJ, Magnasco RP, Becu-Villalobos D, Lacau-Mengido IM, Risco CA, De La Sota RL (2012) Clinical endometritis in an Argentinean herd of dairy cows: risk factors and reproductive efficiency. J Dairy Sci 96:210–218
Hammon DS, Evjen IM, Dhiman TR, Goff JP, Walters JL (2006) Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet Immunol Immunopathol 113:21–29
Hegardt FG (1999) Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J 338:569–582
Heidarpour M, Mohri M, Fallah-Rad AH, Dehghan Shahreza F, Mohammadi M (2014) Hematological changes before and after treatment in dairy cows with clinical and subclinical endometritis. Com Clin Pathol 23:97–101
Herdt TH (2000) Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Vet Clin North Am Food Anim Pract 16:215–230
Hoeben D, Heyneman R, Burvenich C (1997) Elevated levels of betahydroxybutyric acid in periparturient cows and in vitro effect on respiratory burst activity of bovine neutrophils. Vet Immunol Immunopathol 58:165–170
Hoeben D, Monfardini E, Opsomer G, Burvenich C, Dosogne H, De Kruif A, Beckers JF (2000) Chemiluminescence of bovine polymorphonuclear leucocytes during the periparturient period and relation with metabolic markers and bovine pregnancy-associated glycoprotein. J Dairy Res 67:249–259
Huzzey JM, Veira DM, Weary DM, von Keyserlingk MAG (2007) Prepartum behavior and dry matter intake identify dairy cows at risk for metritis. J Dairy Sci 90:3220–3233
Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH (2004) Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 62:9–23
Kaufmann TB, Drillich M, Tenhagen BA, Heuwieser W (2010) Correlations between periparturient serum concentrations of non-esterified fatty acids, betahydroxybutyric acid, bilirubin, and urea and the occurrence of clinical and subclinical postpartum bovine endometritis. BMC Vet Res 6:47–53
Kremer WD, Noordhuizen-Stassen EN, Grommers FJ, Schukken YH, Heeringa R, Brand A, Burvenich C (1993) Severity of experimental Escherichia coli mastitis in ketonemic and nonketonemic dairy cows. J Dairy Sci 76:3428–3436
LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH (2002) Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci 85:2223–2236
LeBlanc SJ (2010) Monitoring metabolic health of dairy cattle in the transition period. J Reprod Dev 56:29–35
National Research Council (2001) Nutrient requirements of dairy cattle, 7th edn. National Academy Press, Washington, DC
Ospina PA, Nydam DV, Stokol T, Overton TR (2010) Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J Dairy Sci 93:1596–1603
Rhoads ML, Gilbert RO, Lucy MC, Butler WR (2004) Effects of urea infusion on the uterine luminal environment of dairy cows. J Dairy Sci 87:2896–2901
Roberts T, Chapinal N, LeBlanc SJ, Kelton DF, Dubuc J, Duffield TF (2012) Metabolic parameters in transition cows as indicators for early-lactation culling risk. J Dairy Sci 95:3057–3063
Rowlands GJ, Lucey S, Russell AM (1986) Susceptibility to disease in the dairy cow and its relationship with occurrences of other diseases in the current of preceding lactation. Prev Vet Med 4:223–234
Salasel B, Mokhtari A, Taktaz T (2010) Prevalence, risk factors for and impact of subclinical endometritis in repeat breeder dairy cows. Theriogenology 74:1271–1278
Scalia D, Lacetera N, Bernabucci U, Demeyere K, Duchateau L, Burvenich C (2006) In vitro effects of nonesterified fatty acids on bovine neutrophils oxidative burst and viability. J Dairy Sci 89:147–154
Seifi HA, Dalir B, Farzaneh N, Mohr M, Gorji-Dooz M (2007) Metabolic changes in cows with or without retained fetal membranes in transition period. J Vet Med 54:92–97
Seifi HA, LeBlanc SJ, Leslie KE, Duffield TF (2011) Metabolic predictors of post-partum disease and culling risk in dairy cattle. Vet J 188:216–220
Senosy WS, Izaike Y, Osawa T (2012) Influences of metabolic traits on subclinical endometritis at different intervals postpartum in high milking cows. Reprod Dom Anim 47(4):666–674
Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ (2009) Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod 81:1025–1032
Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO (2006) Defining postpartum uterine disease in cattle. Theriogenology 65:1516–1530
Soloni FG, Sardina LC (1973) Colorimetric micro determination of free fatty acids. Clin Chem 19:419–424
Van Soest PJ (1994) Nutritional ecology of the ruminant, 2nd edn. Comstock Publishing, Ithaca, New York Associates
Vazquez-Anon M, Bertics S, Luck M, Grummer RR, Pinheiro J (1994) Peripartum liver triglyceride and plasma metabolites in dairy cows. J Dairy Sci 77:1521–1528
Visek WJ (1984) Ammonia: its effects on biological systems, metabolic hormones, and reproduction. J Dairy Sci 67:481–498
Walsh RB, Walton JS, Kelton DF, LeBlanc SJ, Leslie KE, Duffield TF (2007) The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J Dairy Sci 90:2788–2796
Williams EJ, Fischer DP, Pfeiffer DU, England GCW, Noakes DE, Dobson H, Sheldon IM (2005) Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 63:102–117
Acknowledgements
The author wishes to thank the owner and staff of the dairy farm for their kind support and collaboration. The author thanks Azar Laboratory for the measurement of metabolites of the present project.
Funding
This study was funded by Research Deputy of Urmia University (grant no. 012.D.93).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Eslami, M. Alterations of non-esterified fatty acids, β-hydroxybutyric acid, urea, and bilirubin traits in clinical endometritis cows following treatment. Comp Clin Pathol 27, 173–179 (2018). https://doi.org/10.1007/s00580-017-2574-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2574-4