Abstract
Trypanosoma evansi causes significant mortality in dogs. Diagnosis of T. evansi infection can be done by different diagnostic methods including wet blood film examination, stained blood smear examination, advanced serological and molecular tests. In the present study, colloidal dye immunobinding technique was developed for diagnosis of T. evansi in dogs. In this technique, the whole cell lysate antigen was coated on the nitrocellulose membrane of the flow-through device. Protein A colloidal gold was used as detector. The evaluation of the technique was performed by comparing the known positive T. evansi serum collected from dogs and known negative serum. The assay has been validated with wet blood film examination. The present test is an acceptable alternative for use in clinical laboratories which lacks the specialized equipment for screening of T. evansi infection in dogs.
Similar content being viewed by others
Introduction
Trypanosomosis is a disease caused by a flagellate protozoan known as Trypanosoma evansi, transmitted by haematophagous insects. The parasite showed a large diversity of mammalian hosts and is an important disease-producing agent throughout the tropical and subtropical areas of the world (Herrera et al. 2004). Dogs may show clinical changes such as weight loss, progressive weakness, anorexia, intermittent fever, conjunctivitis, swelling of limbs and increased size of superficial lymph nodes (Reddy et al. 2014). Trypanosomosis has received intense consideration and attention due to its disastrous effects on healthy working animals. T. evansi makes an animal unable to perform its duty and causes anaemia, tissue level changes in the organs and abortions in pregnant animals, and gradually, the victim moves towards death (Sivajothi et al. 2013a, 2014a, 2015).
A variety of diagnostic tests are available, and researchers are still trying to improve existing tests and to develop new ones. Current diagnostic tests vary in their sensitivity and specificity. The simplest techniques are examination of wet, thick or thin films of fresh blood; the diagnostic sensitivity of these methods is generally low but depends on the examiner’s experience and the level of parasitaemia. Others include parasite concentration techniques, animal inoculation test, serological tests like indirect fluorescent antibody test, antibody-detection enzyme-linked immune sorbent assay, card agglutination test and polymerase chain reaction (OIE 2008, Sivajothi et al. 2004b).
However, the application of serological tests were not extended to the field and limited to well-equipped laboratories. Owing to these facts and wide geographical distribution of T. evansi, mostly in developing countries, development of rapid, simple-to-perform and cost-effective diagnostic test for the early detection of T. evansi infection using fewer reagents is inevitable; colloidal dye immunobinding assays were reported as inexpensive, simple and field applicable. Previously, Sivajothi et al. (2013b) standardized colloidal dye immunobinding assay for diagnosis of T. evansi infection in domestic animals in Andhra Pradesh. The present study was undertaken to standardize the rapid serodiagnosis of T. evansi in dogs by colloidal dye immunobinding assay and determine the reliability of this test was compared with the microscopic examination of wet blood film, for the diagnosis of T. evansi infection in dogs.
Materials and methods
Blood and serum samples
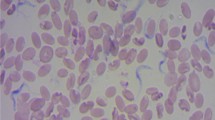
A total of 269 dogs of either sex and all ages suspected for T. evansi infection were selected from different localities in Rayalaseema region of Andhra Pradesh. Dogs showing the signs of lymph adenopathy, chronic emaciation, fever, corneal opacity and hepatitis were included in the present study. Collected blood was stored in separate vials with and without addition of 10 % ethylenediaminetetraacetic acid (EDTA). The blood with anti-coagulant was used to detect T. evansi by wet blood film examination (WBF) (Sivajothi et al. 2013b). The type of organism was diagnosed based on the morphological studies of the stained blood smears (Fig. 1). The blood without anti-coagulant was used for the serum collection. The serum was separated and transferred into sterilized vials after adding one drop of 1:10,000 sodium azide solution and stored at −20 °C till used.
Antigen preparation
T. evansi parasites were collected from the infected dogs and multiplied in the Wister rats for antigen preparation. T. evansi parasites were separated from the blood of experimentally infected Wister rats at high parasitaemia by DEAE (diethyl amino-ethyl cellulose) anion-exchange column chromatography method. Separated trypanosomes were washed twice in PSG (PBS, pH 8.0 with glucose at 1:1) by repeated centrifugation. The purified parasites were then sonicated at 150 W for 3–4 cycles of 30 s each by ultrasonic disintegrator. The sonicated material was centrifuged at 2400 × g for 20 min at 4 °C. The collected supernatant was designated as purified whole cell lysate T. evansi antigen (WCL Ag), and it was partially purified after precipitating with 50 % saturated ammonium sulphate followed by extensive dialysis against PBS, pH 7.4. The protein content of WCL Ag was estimated and was adjusted to 1.0 mg/ml in PBS, pH 8.0, and stored at −20 °C in 1.0-ml aliquots (Sivajothi et al. 2014c).
Raising of hyper immune sera
The hyper immune serum (HIS) was raised in two healthy New Zealand white rabbits. Pre-immunized serum of these experimental rabbits was also stored at −20 °C till use as negative control serum for standardization of colloidal dye immunobinding assay (CDIA) in the present investigation (Sivajothi et al. 2014c).
Development of CDIA
The test was performed in a flow-through module, in which the whole cell lysate antigen of T. evansi-coated nitrocellulose membranes was pressed tightly to a water-absorbing pad.
Test principle
In the assay, antibodies in the serum sample are captured by the antigen of T. evansi spotted on to nitrocellulose membrane mounted on a flow-through test device that serve as the assay capture matrix. The bound antibodies are visualized by the addition of protein A colloidal gold conjugate, which served as antigen-antibody-detecting reagent imparting pink colour to the membrane as a dot. Schematic diagram of colloidal dye immunobinding assay for detection of T. evansi antibodies was mentioned in the Fig. 2.
Detection of T. evansi antibodies by CDIA
The nitrocellulose membrane (M/s mdi, Ambala Cantt, India) was placed above the absorbent pads in a flow-through module. One microlitre (1 g/dl) of whole cell lysate antigen was placed at one end of the module (T side), and 1 μl of known negative serum was placed at the other end (C side), which acted as reagent control. The membrane was dried in an incubator at 37 °C for 1 h or overnight at room temperature, to which 100 μl of wash buffer (25 mM PBS pH 7.0 containing 1 % BSA and 0.05 % Tween 20) was added and allowed to be absorbed through the membrane. Then, 100 μl of test serum diluted in wash buffer (1 in 10) was added and allowed to be absorbed through the membrane. Following this, the membrane was washed with wash buffer. Thereafter, 100 μl of wash buffer diluted (1 in 2) protein A colloidal gold conjugate was added. The appearance of two red colour dots in the ‘T’ and ‘C’ sides of the test window indicated a positive reaction (Fig. 2), and the absence of a red colour dot in the T side of the test window indicated a negative reaction. The test was considered invalid if there were no red dots in the window.
Screening of blood and serum samples
A total of 269 blood and serum samples were tested simultaneously with CDIA and wet blood film examination. The assay (CDIA) has been validated with wet blood film examination.
Results and discussion
The uses of colloidal gold conjugates include the following: as markers for electron microscopy, immunoassay’s, microarray’s and light microscopy (Schultz 2003). Gold markers are the mostly used labels in lateral-flow assays because they have the ability to bind proteins noncovalently. Once bound, the gold will not change the protein’s bioactivity (Wang et al. 2005). It is easy to prepare, it usually has small enough particle size and its red colour can be seen easily against the white background. The evaluation of the result can be made by visual assessment or by a suitable measurement device.
Colloidal dye immunobinding assay (CDIA) was developed to detect circulatory antibodies of T. evansi in the sera of dogs. Checker-board titrations were carried out with various concentrations of antigen, protein A colloidal dye conjugate and known positive and negative sera for T. evansi antibodies to optimize the CDIA. The assay was optimized by using 1 μg/1 μl concentration of WCL Ag, 1:10 dilution of test sera and 1:2 dilution of colloidal dye protein A conjugate (Fig. 3).
In the present study, 269 serum samples were screened by CDIA which were already declared 6 positive by WBF. CDIA found 39 positive for T. evansi antibodies out of 269 sera samples. The presence of red colour dot on the T side and C side indicates positive test (Fig. 4(1)). The presence of red colour dot on the C side indicates negative test (Fig. 4(2)).
Diagnostic methods available so far for detection of T. evansi suffer from one or the other limitations of sensitivity, specificity, cost-effectiveness, field adoptability, rapidity etc. WBF and stained blood smear examination, the commonly used field tests for detection of T. evansi, suffer with least sensitivity (Sivajothi et al. 2012). Animal inoculation methods appear to be more sensitive but are laborious and not useful for immediate diagnosis. CDIA developed in the present study showed high sensitivity and specificity in detection of T. evansi when compared with routinely used WBF method. The CDIA is very simple to perform and can get the result within a few minutes. Further, neither technical expertise nor sophisticated instruments are required for conducting the test. The results can be interpreted visually as the colloidal dye imparts colour in case of the positive test serum only. The colloidal dye conjugate used in CDIA reported to be more stable under field conditions and can be stored at room temperature for at least 6 months (Xiao et al. 2003). The colloidal dye protein A conjugates are less expensive than enzyme conjugates. Protein A colloidal gold conjugate imparts pink colour only to the captured antibodies in the test serum by specific/corresponding antigen spotted on to a nitro-cellulose membrane. In case of negative-test sera samples, there is no chance of availability of the captured antibodies on nitro-cellulose membrane and, hence, no colour formation.
Conclusion
In the present study, we standardized colloidal dye immunobinding assay (the rapid serodiagnostic test ) for easy diagnosis of T. evansi infection in dogs at field level.
References
Herrera HM, Davila AM, Norek A, Abreu UG, Souza SS, Andréa OS, Jansen AM (2004) Enzootiology of Trypanosoma evansi in Pantanal, Brazil. Vet Parasitol 125(3-4):263–275
O.I.E (2008) Trypanosoma evansi infections (including surra) In: OIE terrestrial manual 2008. Office International des Epizooties World Health Organization for Animal Health, Paris, pp 352–360
Reddy BS, Kumari KN, Sivajothi S, Rayulu (2014) Haemato-biochemical and thyroxin status in Trypanosoma evansi infected dogs. J Parasit Dis. doi:10.1007/s12639-014-0531-6, 2014
Schultz DA (2003) Plasmon resonant particles for biological detection. Curr Opin Biotechnol 14:13–22
Sivajothi S, Rayulu VC, Reddy BS (2012) Development of slide enzyme linked immunosorbent assay (SELISA) for detection of Trypanosoma evansi infection in bovines. J Adv Vet Res 2:15–17
Sivajothi S, Rayulu VC, Reddy BS (2013a) Haematological and biochemical changes in experimental Trypanosoma evansi infection in rabbits. J Parasit Dis. doi:10.1007/s12639-013-0321-6
Sivajothi S, Rayulu VC, Malakondaiah P, Sreenivasulu D (2013b) Colloidal dye immunobinding assay for detection of Trypanosoma evansi antibodies in animals. Int J Livest Res 3(3):48–56
Sivajothi S, Rayulu VC, Sujatha K, Reddy BS (2014a) Study of histopathological changes in experimental Trypanosoma evansi infected rats. Proc Zool Soc. doi:10.1007/s12595-014-0104-9
Sivajothi S, Rayulu VC, Reddy BS (2014b) Detection of Trypanosoma evansi by different methods in bovines in Andhra Pradesh. J Adv Parasitol 1(3):35–38
Sivajothi S, Rayulu VC, Malakondaiah P, Sreenivasulu D (2014c) Diagnosis of Trypanosoma evansi in bovines by indirect ELISA. J Parasit Dis. doi:10.1007/s12639-014-0465-z
Sivajothi S, Reddy BS, Reddy YVP, Rayulu VC (2015) Abortion due to Trypanosomosis in a buffalo. Inventi Impact Infect 2015(1):1–2
Wang S, Zhang C, Wang J, Zhang Y (2005) Development of colloidal gold-based flow-through and lateral-flow immunoassays for the rapid detection of the insecticide carbaryl. Anal Chim Acta 546:161–166
Xiao X, Wan T, Tian Z (2003) Development of rapid, sensitive, dye immunoassay for Schistosomosis diagnosis: a colloidal dye immunofiltration assay. J Immunol Methods 280:49–57
Acknowledgments
The authors express special thanks to the blind peer-reviewed procedures and to the reviewers who suggested them good valuable corrections to improve their article. The authors acknowledge the authorities of Sri Venkateswara Veterinary University, Tirupati, for providing facilities to carry out this research. The authors are also thankful to the veterinary doctors who assisted in the collection of blood samples from the dogs at field level.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivajothi, S., Rayulu, V.C. & Reddy, B.S. Rapid serodiagnosis of Trypanosoma evansi in dogs by colloidal dye immunobinding assay. Comp Clin Pathol 24, 1497–1500 (2015). https://doi.org/10.1007/s00580-015-2106-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-015-2106-z