Abstract
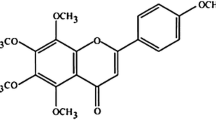
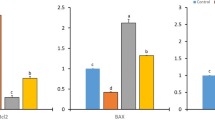
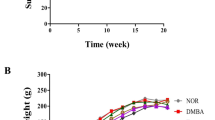
Oxidative stress resulting from increased free radical generation plays an important role in carcinogenesis. The present study investigates the therapeutic efficacy of Semecarpus anacardium Linn nut milk extract (SA) against lipid peroxidation (LPO) and antioxidant status in spleen and thymus of experimental animals. Adult female albino rats of Sprague–Dawley strain weighing 170–190 g were used for the study and were divided into four groups. Group I control animals received standard pellet diet and water ad libitum. Group II rats were induced with 7,12-dimethyl benz[a]anthracene (DMBA) (25 mg in 1 ml olive oil) by gastric incubation, whereas another set of DMBA-induced rats were treated with SA (200 mg/kg body weight/day) in olive oil orally by gastric incubation for 14 days (group III). Group IV rats served as SA-treated control animals. At the end of the experimental period, the rats were anaesthetised and killed. Organs like spleen and thymus were isolated and used for biochemical measures and histology studies. In cancer condition, the LPO was increased and antioxidant levels were decreased in the spleen and thymus of cancer-induced rats. Treatment with SA decreased LPO and increased antioxidant status in rats induced with DMBA. The results obtained depict the potency of drug’s antioxidant property by depleting lipid peroxide levels in spleen and thymus and thus its role in enhancing the immune function.




Similar content being viewed by others
References
Acker SABEV, Berg DJVD, Tromp MNJL, Griffioen DH, Bennekom WP, Vigh WJFV, Bast A (1996) Structural aspects of anti-oxidant activity of flavonoids. Free Rad Biol Med 20:331–342
ACS (2009) Breast cancer facts and figures
Argiles JM, Aczon-Bieto J (1998) The metabolic environment of cancer. Mol Cell Biochem 81:3–17
Arul B, Kothai R, Christina AJ (2004) Hypoglycemic and antihyperglycemic effect of Semecarpus anacardium Linn in normal and streptozotocin induced diabetic rats. Methods Find Exp Clin Pharmacol 26:759–762
Arulkumaran S, Ramprasath VR, Shanthi P, Sachdanandam P (2006) Restorative effect of Kalpaamruthaa, an indigenous preparation, on oxidative damage in mammary gland mitochondrial fraction in experimental mammary carcinoma. Mol Cell Biochem 291:77–82
Babcor BM, Kipers RS, Curnutte JT (1973) The production by leucocytes of superoxide dismutase, a potential bactericidal agent. J Clin Invest 52:741–744
Baehner RI, Boxer LA, Allen JM, Davis J (1997) Antioxidant as a basis for altered function of polymorphonuclear leukocytes. Blood 50:327
Barber MD, Fearon KC, Tisdale MJ, Mc Millan DC, Ross JA (2001) Effect of a fish oil-enriched nutritional supplement on metabolic mediators in patients with pancreatic cancer cachexia. Nutr Cancer 40:110–124
Bendich A (1990) Antioxidant vitamins and their functions in immune responses. Adv Exp Med Biol 262:35–55
Ben-Hur H, Kossoy G, Schneider DF, Zandbank J, Zusman I (2002) Response of the immune system of mammary tumor-bearing rats to cyclophosphamide and soluble low-molecular mass tumor-associated antigens: the spleen and lymph nodes. Int J Mol Med 3:311–316
Ben-Hur H, Kossoy G, Sanko H, Geva D, Shumlin N, Zusman I, Elhayany A (2004) Response of T- and B-lymphocytes in the spleen of mammary tumor-bearing rats to treatment with tamoxifen and soluble tumor-associated antigens. Oncol Rep 1:181–185
Bhuvaneswari V, Velmurugan B, Abraham SK, Nagini S (2004) Tomato and garlic by oral gavage modulate 7, 12 dimethyl beng [a] anthracene-induced genotoxicity and oxidative stress in mice. Braz J Med Biol Res 37:1029–1034
Bohm H, Boeing H, Hempel J, Raab B, Kroke A, Ernahrungswiss Z (1998) Flavanols, flavones and anthocyanins as natural antioxidants of food and their possible role in the prevention of chronic diseases. Z Ernahrungswiss 37:147–163
Chance B, Greenstien DS, Roughten RJW (1952) The mechanism of catalase action-steady state analysis. Arch Biochem Biophys 37:301–339
Chattopadhyaya MK, Khare RL (1969) Isolation of anacardic acid from Semecarpus anacardium Linn. and study of its anthelmintic activity. Ind J Pharm 31:104–105
Chitinis P, Bhatia KG, Phatak MK, Kesava Rao KV (1980) Antitumour activity of the extract of Semecarpus anacardium Linn nuts in experimental tumour models. Ind Exp Biol 18:6–8
Choi YJ, Kang JS, Park JHY, Lee YJ, Choi JS, Kang YH (2003) Polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide treated human vascular endothelial cells The American Society for Nutritional Sciences. J Nutr 133:985–991
Corzo CA, Nagaraj S, Kusmartsev S, Gabrilovich D (2007) Role of reactive oxygen species in immune suppression in cancer. J Immunol 178:49.12
Costa I, Solanas M, Escrich E (2002) Histopathologic characterization of mammary neoplastic lesions induced with 7, 12 dimethylbenz(alpha)anthracene in the rat: a comparative analysis with human breast tumours. Arch Pathol Lab Med 126:915–927
De la Fuente M (2002) Effects of antioxidants on immune system ageing. Eur J Clin Nutr 56:S5–S8
Dean RT, Fu S, Stocker R, Davies MJ (1997) Biochemistry and pathology of radical mediated protein oxidation. Biochemistry 324:1–18
Devasagayam TPA, Tarachand V (1987) Decreased LPO in the rat kidney during gestation. Biochem Biophys Res Commun 145:469–474
Droge W, Breitkreutz R (2000) Glutathione and immune function. Proc Nutr Soc-Engl Scot 59:595–600
Escrich E, Moral R, Garcá G, Costa I (2004) Identification of novel differentially expressed genes by the effect of a high-fat n-6 diet in experimental breast cancer. Mol Carcinog 40:73–78
Fernandez-Checa JC, Yi J, Ruiz CG, Ookhtens M, Kaplowitz N (1996) Plasma membrane and mitochondrial transport of hepatic reduced glutathione. Semin Liver Dis 16:147–158
Formulary of Siddha Medicine (1972) 2nd edn. Madras: Indian Medicine Practitioners Co-operative Pharmacy and Stores Ltd: 197
Fraga BM (1987) Natural sesquiterpenoids. Nat Prod Rep 4:473–498
Frei B, England L, Ames BN (1986) Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 86:6377–6381
Gao J, Laver FT, Dunaway S, Burchiel SW (2005) Cytochrane p450 [b] is required for 7, 12 Dimethyl beng (a) anthracene (DMBA) induced spleen cell immunotoxicity. Toxicol Sci 86:68–74
Ghothoskar SV, Ranadive KJ (1971) Anticancer screening of SAN- AB: An extract of marking nut, Semecarpus anacardium. Indian J Exp Biol 9:372–375
Gonenc A, Ozkan Y, Torun M, Simsek B (2001) Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J Clin Pharm Ther 26:141–144
Halliwell B, Gutteridge JMC (1985) Free radicals and Toxicology In: Free Radicals in Biology and medicine Oxford Larendon Press 1–27
Hua T-J, Shuai X-H, Chen J-R, Wei YY, Zheng R-L (2009) Protective effect of a Potentilla anserine polysaccharide on oxidative damages in mice. Int J Biol Macromol 45:279–283
Huang YL, Sheer JY, Lin TH (1999) Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin Biochem 32:131–136
Indap MA, Ambaye RY, Gokhale SV (1986) Potentiation of activity of anticancer drugs by acetylated oil of Semecarpus anacardium Linn. F. in experimental tumours. Indian Drugs 23:447
Inefers H, Sies H (1988) The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem 174:353–357
Kawamura T, WhisaY AY, Ishimori A, Shinda R, Yokota K (1992) Plasma lipid peroxide in the operation of oesophageal cancer. Rinsho-Ayori 40:881–884
Keit I, Block MD, Boyd B, Gonzalez N, Vojdani A (2002) The immune system in cancer. Integr Cancer Ther 1:294–316
Khanzode SS, Muddeshwar MG, Khanzode SD, Dakhale GN (2004) Antioxidant enzymes and lipid peroxidation in different stages of breast cancer. Free Radic Res 38:81–85
Kritikar KR, Basu BD (1933) In: Basu LM (ed) Indian medicinal plants, 2nd edn. M/S Bisheh Sigh, Mahendra Palsingh, Allahabad, 667–670
Leij-Halfwerk S, Dagnelie PC, van Den Berg JOW, Wattimena JD, Hordijk-Luijk CH, Wilson JP (2000) Weight loss and elevated gluconeogenesisfrom alanine in lung cancer patients. Am J Clin Nutr 71:583–589
Linden M, Hakansson L, Ohlsson K, Sjodin K, Tegner H, Tunek A, Venge P (1989) Glutathione in bronchoalveolar lavage fluid from smokers is related to humoral markers of inflammatory cell activity. Inflammation 13:651–658
Lopez DM, Charyulu V, Adkins B (2002) Influence of breast cancer on thymic function in mice. J Mammary Gland Biol Neoplasia 2:191–199
Lowry OH, Rosenbrough NJ, Farn AL, Randall RJ (1951) Protein measurement with folin’s phenol reagent. J Biol Chem 193:265–275
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
McCord JM, Keele BB, Fridorich I (1976) An enzyme based theory of obligate anaerobics the physiological functions of superoxide dismutase. R Voc Natl Acad Sci USA 68:1014–1017
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione Stransferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Nair PKR, Melnick SJ, Wnuk SF, Rapp M, Escalon E, Ramachandran C (2009) Isolation and characterization of an anticancer catechol compound from Semecarpus anacardium. J Ethnopharmacol 3:450–456
Okawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Omaye ST, Turnbull JD, Sauberlich HE (1979) Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol 62:3–11
Pandey R, Maurya R, Singh G, Sathiamoorthy B, Naik S (2005) Immunosuppressive properties of flavonoids isolated from Boerhaavia diffusa Linn. Int Immunopharmacol 3:541–553
Perin N, Notani N (2001) Global variation in cancer incidence and mortality. Curr Sci 81:5–10
Prakasa Rao NS, Ramachandra Row L, Brown RT (1973) Phenolic constituents of Semecarpus anacardium. Phytochemicals 12:671–680
Quaife MC, Dju MY (1948) Chemical estimation of vitamin E in tissues and the α-tocopherol content of normal tissues. J Biol Chem 180:263–272
Rai MK, Pandey AK, Acharya D (2000) Ethno-medicinal plants used by Gond tribe of Bhanadehi, District Chhindwara, Madhya Pradesh. J Non-Timber Forest 7:237–241
Ramprasath VR, Shanthi P, Sachdanandam P (2005) Evaluation of antioxidant effect of Semecarpus anacardium Linn. nut extract on the components of immune system in adjuvant arthritis. Vasc Pharmacol 4:179–186
Ramprasath VR, Shanthi P, Sachdanandam P (2006) Immunomodulatory and anti-inflammatory effects of Semecarpus anacardium Linn. Nut milk extract in experimental inflammatory conditions. Biol Pharma Bull 4:693–700
Roitt I, Brostoff J, Male D (1996) Immunology, 4th edn. Mosby, London
Rola-Plezczunski M (1985) Imunoregulation by leukotrienes and other lipoxygenase metabolites. Immunol Today 6:302–307
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hekstra WG (1973) Selenium, biochemical role as a component of glutathione peroxidase purification and assay. Science 179:588–590
Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F (1995) Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med 19:481–486
Sanz MJ, Fernandez ML, Cyuero M, Terencio MC (1994) Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotics 24:689–699
Satyavati GV, Prasad DN, Das PK, Singh HD (1969) Anti-inflammatory activity of Semecarpus anacardium Linn. A preliminary study. Ind J Physiol Pharmacol 13:37
Selvam C, Jachak SM (2004) A cyclooxygenase (COX) inhibitory biflavonoid from the seeds of Semecarpus anacardium. J Ethnopharmacol 95:209–212
Shakti Prasad P, Mazumder PM (2010) Therapeutic potential of Dendrophthoe falcata (L.f) Ettingsh on 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in female rats: effect on antioxidant system, lipid peroxidation, and hepatic marker enzymes. Comp Clin Pathol. doi:10.1007/s00580-010-1008
Sinha A (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Leong SP, Shen ZZ, Liu T-J, Agarwal G, Tajima T, Paik N-S, Sandelin K, Derossis A, Cody H, Foulkes WD (2010) Is breast cancer the same disease in Asian and Western countries? World J Surg 34:2308–2324
Sujatha V, Sachdanandam P (2002) Recuperative effect of Semecarpus anacardium Linn. nut milk extract on carbohydrate metabolizing enzymes in experimental mammary carcinoma-bearing rats. Phytother Res 16:14–18
Tanaka H, Sugiura H, Inaba R, Nishikawa A, Murakami A, Koshimizu K, Ohigashi H (1999) Immunomodulatory action of citrus auraptene on macrophage functions and cytokine production of lymphocytes in female BALB/c mice. Carcinogenesis 20:1471–1476
Hoffeld JT (1981) Agents which block membrane lipid peroxidation enhance mouse spleen cell immune activities in vitro: relationship to the enhancing activity of 2-mercaptoethanol. Eur J Immunol 5:371–376
US Cancer Statistics Working Group (2009) United States Cancer Statistics: 1999–2005 Incidence and Mortality Web-based Report Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention. National Cancer Institute, Washington
Vijayalakshmi T, Muthulakshmi V, Sachdanandam P (2000) Toxic studies on biochemical parameters carried out in rats with Serankottai nei, a Siddha drug-milk extract of Semecarpus anacardium nut. J Ethnopharmacol 69:9–15
Vuokko L, Kinnula, Crapo JD (2004) Superoxide dismutases in malignant cells and human tumors. Free Radic Biol Med 36:718–744
Welsch CW (1985) Factors affecting the growth of carcinogen induced mammary carcinomas. A review and tribute to Charles Brenton Huggins. Cancer Res 45:3415–3443
Younes M, Craig G, Stacey NH (1986) Cell-mediated cytotoxicity by natural killer cells, lipid peroxidation and glutathione. Experientia 42:1257–1262
Zhai S, Dai R, Friedman FK, Vestal RE (1998) Comparative inhibition of human cytochromes P450 1A1 and 1A2 by flavonoid. Drug Metab Dispos 26:989–992
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, H.B.H., Vinayagam, K.S., Madan, P. et al. Modulatory effect of Semecarpus anacardium against oxidative damages in DMBA-induced mammary carcinogenesis rat model. Comp Clin Pathol 21, 1275–1284 (2012). https://doi.org/10.1007/s00580-011-1278-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-011-1278-4