Abstract
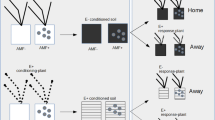
Plant-soil feedback (PSF) describes the process whereby plant species modify the soil environment, which subsequently impacts the growth of the same or another plant species. Our aim was to explore PSF by two maize varieties (a landrace and a hybrid variety) and three arbuscular mycorrhizal fungi (AMF) species (Funneliformis mosseae, Claroideoglomus etunicatum, Gigaspora margarita, and the mixture). We carried out a pot experiment with a conditioning and a feedback phase to determine PSF with different species of AMF and with a non-mycorrhizal control. Sterilized soil was conditioned separately by each variety, with or without AMF; in the feedback phase, each soil community was used to grow each in its “home” soil and in the “away” soil. Plant performance was assessed as shoot biomass, phosphorus (P) concentration and P content, and fungal performance was assessed as mycorrhizal colonization and hyphal length density. Both maize varieties were differentially influenced by AMF in the conditioning phase. In the feedback phase, PSF was generally negative for non-mycorrhizal plants or when plants were colonized by G. margarita, whereas PSF was positive in the other three AMF treatments. When plants were grown on home soil, hyphal length density was larger than on away soil. We conclude that different maize varieties can strengthen positive plant-soil feedback for themselves through beneficial mutualists for themselves, but not across the maize varieties.




Similar content being viewed by others
References
Angelard C, Tanner CJ, Fontanillas P, Niculita-Hirzel H, Masclaux F, Sanders IR (2014) Rapid genotypic change and plasticity in arbuscular mycorrhizal fungi is caused by a host shift and enhanced by segregation. Isme J 8:284–294
Bever JD (2002a) Host-specificity of AM fungal population growth rates can generate feedback on plant growth. Plant Soil 244:281–290
Bever JD (2002b) Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proc R Soc B Biol Sci 269:2595–2601
Bever JD (2003) Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol 157:465–473
Bever JD, Westover KM, Antonovics J (1997) Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J Ecol 85:561–573
Bever JD, Richardson SC, Lawrence BM, Holmes J, Watson M (2009) Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol Lett 12:13–21
Bezemer T, Lawson CS, Hedlund K, Edwards AR, Brook AJ, Igual JM, Mortimer SR, van der Putten WH (2006) Plant species and functional group effects on abiotic and microbial soil properties and plant–soil feedback responses in two grasslands. J Ecol 94:893–904
Brinkman EP, van der Putten WH, Bakker EJ, Verhoeven KJF (2010) Plant-soil feedback: experimental approaches, statistical analyses and ecological interpretations. J Ecol 98:1063–1073
Bukowski AR, Petermann JS (2014) Intraspecific plant-soil feedback and intraspecific overyielding in Arabidopsis thaliana. Ecol Evol 4:2533–2545
Chu Q, Wang XX, Yang Y, Chen FJ, Zhang FS, Feng G (2013) Mycorrhizal responsiveness of maize (Zea mays L.) genotypes as related to releasing date and available P content in soil. Mycorrhiza 23:497–505
Dias T, Dukes A, Antunes PM (2015) Accounting for soil biotic effects on soil health and crop productivity in the design of crop rotations. J Sci Food Agric 95:447–454
Ehinger MO, Croll D, Koch AM, Sanders IR (2012) Significant genetic and phenotypic changes arising from clonal growth of a single spore of an arbuscular mycorrhizal fungus over multiple generations. New Phytol 196:853–861
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant-soil system. Annu Rev Environ Resour 30:75–115
Gubry-Rangin C, Garcia M, Bena G (2010) Partner choice in Medicago truncatula-Sinorhizobium symbiosis. Proc R Soc B Biol Sci 277:1947–1951
Hart MM, Reader RJ (2002) Does percent root length colonization and soil hyphal length reflect the extent of colonization for all AMF? Mycorrhiza 12:297–301
Heath KD, Tiffin P (2009) Stabilizing mechanisms in a legume-rhizobium mutualism. Evolution 63:652–662
Hendriks M, Mommer L, Caluwe H, Smit-Tiekstra AE, Putten WH, Kroon H (2013) Independent variations of plant and soil mixtures reveal soil feedback effects on plant community overyielding. J Ecol 101:287–297
Hol W, De Boer W, Termorshuizen AJ, Meyer KM, Schneider JH, Van Dam NM, Van Veen JA, Van der Putten WH (2010) Reduction of rare soil microbes modifies plant–herbivore interactions. Ecol Lett 13:292–301
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 2. Hyphal transport of 32P over defined distances. New Phytol 120:509–516
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bucking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Koyama A, Pietrangelo O, Sanderson L, Antunes PM (2017) An empirical investigation of the possibility of adaptability of arbuscular mycorrhizal fungi to new hosts. Mycorrhiza 27:553–563
Kulmatiski A, Beard KH, Stevens JR, Cobbold SM (2008) Plant-soil feedbacks: a meta-analytical review. Ecol Lett 11:980–992
Kulmatiski A, Beard KH, Heavilin J (2012) Plant-soil feedbacks provide an additional explanation for diversity-productivity relationships. Proc R Soc B Biol Sci 279:3020–3026
Kuyper TW, De Goede G (2013) Interactions between higher plants and soil-dwelling organisms. In: Van der Maarel E, Franklin J (eds) Vegetation ecology, 2nd editn. John Wiley & Sons, pp. 260–284
Li H, Zhang D, Wang X, Li H, Rengel Z, Shen J (2018) Competition between Zea mays genotypes with different root morphological and physiological traits is dependent on phosphorus forms and supply patterns. Plant Soil 434:125–137
Ma HK, Pineda A, van der Wurff AWG, Raaijmakers C, Bezemer TM (2017) Plant-soil feedback effects on growth, defense and susceptibility to a soil-borne disease in a cut flower crop: species and functional group effects. Front Plant Sci 8
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, Carneiro NP, Guimaraes CT, Schaffert RE, Sa NMH (2009a) Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian cerrado biome. Soil Biol Biochem 41:1782–1787
Oliveira CA, Sá NM, Gomes EA, Marriel IE, Scotti MR, Guimarães CT, Schaffert RE, Alves V (2009b) Assessment of the mycorrhizal community in the rhizosphere of maize (Zea mays L.) genotypes contrasting for phosphorus efficiency in the acid savannas of Brazil using denaturing gradient gel electrophoresis (DGGE). Appl Soil Ecol 41:249–258
Redecker D, Schussler A, Stockinger H, Sturmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531
Sikes BA, Cottenie K, Klironomos JN (2009) Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J Ecol 97:1274–1280
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, New York, pp 1–769
Trouvelot A, Kough J, Gianiazzi-Pearson V (1986) Mesure du taux de mycorrhization VA d’un système radiculaire. Recherche de methodsd’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological genetical aspects of mycorrhizae. INRA Press, Paris, pp 217–221
Umbanhowar J, McCann K (2005) Simple rules for the coexistence and competitive dominance of plants mediated by mycorrhizal fungi. Ecol Lett 8:247–252
Van der Heijden MGA (2002) Arbuscular mycorrhizal fungi as a determinant of plant diversity: in search of underlying mechanisms and general principles. In: Van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer-Verlag, Berlin, pp 243–265
Wang XX, Hoffland E, Feng G, Kuyper TW (2017) Phosphate uptake from phytate due to hyphae-mediated phytase activity by arbuscular mycorrhizal maize. Front Plant Sci 8
Wehner J, Antunes PM, Powell JR, Mazukatow J, Rillig MC (2010) Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiologia 53:197–201
Zhou XG, Liu J, Wu FZ (2017) Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 415:507–520
Acknowledgements
We are grateful to editor David Janos and two anonymous reviewers for constructive criticism on an earlier version of this manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (U1703232) and National Key R&D Program of China (2017YFD0200200).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 472 kb)
Rights and permissions
About this article
Cite this article
Wang, XX., Hoffland, E., Mommer, L. et al. Maize varieties can strengthen positive plant-soil feedback through beneficial arbuscular mycorrhizal fungal mutualists. Mycorrhiza 29, 251–261 (2019). https://doi.org/10.1007/s00572-019-00885-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-019-00885-3