Abstract
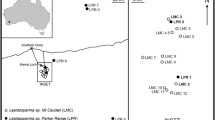
To understand the reproduction of the pioneer ectomycorrhizal fungi Laccaria amethystina and Laccaria laccata in a volcanic desert on Mount Fuji, Japan, the in situ genet dynamics of sporocarps were analysed. Sporocarps of the two Laccaria species were sampled at fine and large scales for 3 and 2 consecutive years, respectively, and were genotyped using microsatellite markers. In the fine-scale analysis, we found many small genets, the majority of which appeared and disappeared annually. The high densities and annual renewal of Laccaria genets indicate frequent turnover by sexual reproduction via spores. In the large-scale analysis, we found positive spatial autocorrelations in the shortest distance class. An allele-clustering analysis also showed that several alleles were distributed in only a small, localised region. These results indicate that Laccaria spores contributing to sexual reproduction may be dispersed only short distances from sporocarps that would have themselves been established via rare, long-distance spore dispersal. This combination of rare, long-distance and frequent, short-distance Laccaria spore dispersal is reflected in the establishment pattern of seeds of their host, Salix reinii.




Similar content being viewed by others
References
Adams RI, Hallen HE, Pringle A (2006) Using the incomplete genome of the ectomycorrhizal fungus Amanita bisporigera to identify molecular polymorphisms in the related Amanita phalloides. Mol Ecol Notes 6:218–220
Beiler KJ, Durall DM, Simard SW, Maxwell SA, Kretzer AM (2010) Architecture of the wood-wide web: Rhizopogon spp. genets link multiple Douglas-fir cohorts. New Phytol 185:543–553
Bergemann SE, Miller SL (2002) Size, distribution, and persistence of genets in local populations of the late-stage ectomycorrhizal basidiomycete, Russula brevipes. New Phytol 156:313–320
Bergemann SE, Miller SL, Garbelotto M (2005) Microsatellite loci from Russula brevipes, a common ectomycorrhizal associate of several tree species in North America. Mol Ecol Notes 5:472–474
Bergemann SE, Douhan GW, Garbelotto M, Miller SL (2006) No evidence of population structure across three isolated subpopulations of Russula brevipes in an oak/pine woodland. New Phytol 170:177–184
Carriconde F, Gardes M, Jargeat P, Heilmann-Clausen J, Mouhamadou B, Gryta H (2008) Population evidence of cryptic species and geographical structure in the cosmopolitan ectomycorrhizal fungus, Tricholoma scalpturatum. Micro Ecol 56:513–534
Dahlberg A, Stenlid J (1994) Size, distribution and biomass of genets in populations of Suillus bovinus (L. Fr.) Roussel revealed by somatic incompatibility. New Phytol 128:225–234
Donges K, Schlobinski D, Cremer E, Rexer KH, Kost G (2008) Six newly developed microsatellite markers of Laccaria amethystina, using an improved CSSR approach. Mycol Prog 7:285–290
Douhan GW, Vincenot L, Gryta H, Selosse M-A (2011) Population genetics of ectomycorrhizal fungi: from current knowledge to emerging directions. Fungal Biol 115:569–597
Dunham SM, Kretzer A, Pfrender ME (2003) Characterization of Pacific golden chanterelle (Cantharellus formosus) genet size using co-dominant microsatellite markers. Mol Ecol 12:1607–1618
Dunham SM, O’Dell TE, Molina R (2006) Spatial analysis of within-population microsatellite variability reveals restricted gene flow in the Pacific golden chanterelle (Cantharellus formosus). Mycologia 98:250–259
Fiore-Donno AM, Martin F (2001) Populations of ectomycorrhizal Laccaria amethystina and Xerocomus spp. Show contrasting colonization patterns in a mixed forest. New Phytol 152:533–542
Galante TE, Horton TR, Swaney DP (2011) 95 % of basidiospores fall within 1 m of the cap: a field- and modeling-based study. Mycologia 103:1175–1183
Gherbi H, Delaruelle C, Selosse M-A, Martin F (1999) High genetic diversity in a population of the ectomycorrhizal basidiomycete Laccaria amethystina in a 150-year-old beech forest. Mol Ecol 8:2003–2013
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices version 2.9.3. Available from http://www2.unil.ch/popgen/softwares/fstat.htm
Grubisha LC, Kretzer AM, Bruns TD (2005) Isolation and characterization of microsatellite loci from the truffle-like ectomycorrhizal fungi Rhizopogon occidentalis and Rhizopogon vulgaris. Mol Ecol Notes 5:608–610
Grubisha LC, Bergemann SE, Bruns TD (2007) Host islands within the California Northern Channel Islands create fine-scale genetic structure in two sympatric species of the symbiotic ectomycorrhizal fungus Rhizopogon. Mol Ecol 16:1811–1822
Guidot A, Debaud JC, Marmeisse R (2001) Correspondence between genet diversity and spatial distribution of above- and below-ground populations of the ectomycorrhizal fungus Hebeloma cylindrosporum. Mol Ecol 10:1121–1131
Guidot A, Debaud JC, Effosse A, Marmeisse R (2004) Below-ground distribution and persistence of an ectomycorrhizal fungus. New Phytol 161:539–547
Hirose D, Tokumasu S (2007) Microsatellite loci from the ectomycorrhizal basidiomycete Suillus pictus associated with the genus Pinus subgenus Strobus. Mol Ecol Res 7:854–856
Hirose D, Kikuchi J, Kanzaki N, Futai K (2004) Genet distribution of sporocarps and ectomycorrhizas of Suillus pictus in a Japanese white pine plantation. New Phytol 164:527–541
Hitchcock CJ, Chambers SM, Cairney JWG (2011) Genetic population structure of the ectomycorrhizal fungus Pisolithus microcarpus suggests high gene flow in south-eastern Australia. Mycorrhiza 21:131–137
Hogberg N, Guidot A, Jonsson M, Darhberg A (2009) Microsatellite markers for the ectomycorrhizal basidiomycete Lactarius mammosus. Mol Ecol Res 9:1008–1010
Hortal S, Trocha LK, Murat C, Chybicki IJ, Buee M, Trojankiewicz M, Burczyk J, Martin F (2012) Beech roots are simultaneously colonized by multiple genets of the ectomycorrhizal fungus Laccaria amethystina clustered in two genetic groups. Mol Ecol 21:2116–2129
Ishida TA, Nara K, Tanaka M, Kinoshita A, Hogetsu T (2008) Germination and infectivity of ectomycorrhizal fungal spores in relation to their ecological traits during primary succession. New Phytol 180:491–500
Jany JL, Bousquet J, Khasa DP (2003) Microsatellite markers for Hebeloma species developed from expressed sequence tags in the ectomycorrhizal fungus Hebeloma cylindrosporum. Mol Ecol Res 3:659–661
Jany JL, Bousquet J, Gagne A, Khasa DP (2006) Simple sequence repeat (SSR) markers in the ectomycorrhizal fungus Laccaria bicolor for environmental monitoring of introduced strains and molecular ecology applications. Mycol Res 110:51–59
Kanchanaprayudh J, Lian C, Zhou Z, Hogetsu T, Sihanonth P (2002) Polymorphic microsatellite markers of a Pisolithus sp. from a Eucalyptus plantation. Mol Ecol Notes 2:263–264
Kretzer AM, Molina R, Spatafora JW (2000) Microsatellite markers for the ectomycorrhizal basidiomycete Rhizopogon vinicolor. Mol Ecol 9:1190–1191
Kretzer AM, Dunham S, Molina R, Spatafora JW (2003) Microsatellite markers reveal the below ground distribution of genets in two species of Rhizopogon forming tuberculate ectomycorrhizas on Douglas fir. New Phytol 161:313–320
Kretzer AM, Dunham S, Molina R, Spatafora JW (2005) Patterns of vegetative growth and geneflow in Rhizopogon vinicolor and R. vesiculosus (Boletales, Basidiomycota). Mol Ecol 14:2259–2268
Lian C, Hogetsu T (2002) Development of microsatellite markers in black locust (Robinia pseudoacacia) using dual-suppression-PCR technique. Mol Ecol Notes 2:211–213
Lian C, Hogetsu T, Matsushita N, Guerin-Laguette A, Suzuki K, Yamada A (2003a) Development of microsatellite markers from an ectomycorrhizal fungus, Tricholoma matsutake, by an ISSR-suppression-PCR method. Mycorrhiza 13:27–31
Lian C, Oishi R, Miyashita N, Nara K, Nakaya H, Zhou Z, Wu B, Hogetsu T (2003b) Genetic structure and reproduction dynamics of Salix reinii during primary succession on Mt. Fuji, as revealed by nuclear and chloroplast microsatellite analysis. Mol Ecol 12:609–618
Lian C, Narimatsu M, Nara K, Hogetsu T (2006) Tricholoma matsutake in a natural Pinus densiflora forest: correspondence between above- and below ground genets, association with multiple host trees and alteration of existing ectomycorrhizal communities. New Phytol 171:825–836
Muller LAH, Vangronsveld J, Colpaert JV (2007) Genetic structure of Suillus luteus populations in heavy metal polluted and nonpolluted habitats. Mol Ecol 16:4728–4737
Nara K (2006) Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol 171:187–198
Nara K, Hogetsu T (2004) Ectomycorrhizal fungi on established shrubs facilitate subsequent seedling establishment of successful plant species. Ecology 85:1700–1707
Nara K, Nakaya H, Hogetsu T (2003a) Ectomycorrhizal sporocarp succession and production during early primary succession on Mount Fuji. New Phytol 158:193–206
Nara K, Nakaya H, Wu B, Zhou Z, Hogetsu T (2003b) Underground primary succession of ectomycorrhizal fungi in a volcanic desert on Mount Fuji. New Phytol 159:743–756
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Roy M, Dubois M-P, Proffit M, Vincenot L, Desmarais E, Selosse M-A (2008) Evidence from population genetics that the ectomycorrhizal basidiomycete Laccaria amethystina is an actual multihost symbiont. Mol Ecol 17:2825–2838
Rubini A, Paolocci F, Riccioni C, Vendramin GG, Arcioni S (2005) Genetic and phylogeographic structures of the symbiotic fungus Tuber magnatum. Appl Environ Microbiol 71:6584–6589
Selosse ECMA, Jacquot D, Bouchard D, Martin F, Le Tacon F (1998) Temporal persistence and spatial distribution of an American inoculant strain of the ectomycorrhizal basidiomycete Laccaria bicolor in European forest plantations. Mol Ecol 7:561–573
Selosse MA, Martin F, Le Tacon F (1999) Structure and dynamics of experimentally introduced and naturally occurring Laccaria spp. discrete genotypes in a Douglas fir plantation. Appl Environ Microbiol 65:2006–2014
Smouse PE, Peakall R (1999) Spatial autocorrelation analysis of multi-allele and multi-locus genetic micro-structure. Heredity 82:561–573
Vincenot L, Nara K, Sthults C, Labbe J, Dubois MP, Tedersoo L, Martin F, Selosse MA (2012) Extensive gene flow over Europe and possible speciation over Eurasia in the ectomycorrhizal basidiomycete Laccaria amethystina complex. Mol Ecol 21:281–299
Wadud MA, Lian CL, Nara K, Hogetsu T (2006a) Isolation and characterization of five microsatellite loci in an ectomycorrhizal fungus Laccaria laccata. Mol Ecol Notes 6:700–702
Wadud MA, Lian CL, Nara K, Ishida TA, Hogetsu T (2006b) Development of microsatellite markers from an ectomycorrhizal fungus, Laccaria amethystina, by a dual-suppression-PCR technique. Mol Ecol Notes 6:130–132
Wadud MA, Lian CL, Nara K, Ishida TA, Hogetsu T (2007) Below-ground genet structure of an ectomycorrhizal fungus, Laccaria amethystina in the volcanic desert on Mount Fuji. J Agrofor Environ 1:155–160
Wadud MA, Lian CL, Nara K, Reza MS, Hogetsu T (2008) Below ground genet differences of an ectomycorrhizal fungus Laccaria laccata infecting Salix stands in primary successional stage. J Agrofor Environ 2:1–6
Wahlund S (1928) Zusammensetzung von Populationen und Korrelationserscheinungen vom Standpunkt der Vererbungslehre aus betrachtet. Hereditas 11:65–106
Wu B, Nara K, Hogetsu T (2005) Genetic structure of Cenococcum geophilum populations in primary successional volcanic deserts on Mount Fuji as revealed by microsatellite markers. New Phytol 165:285–293
Xiao Y, Liu W, Dai Y, Fu C, Bian Y (2010) Using SSR markers to evaluate the genetic diversity of Lentinula edodes natural germplasm in China. World J Microbiol Biotech 26:527–5326
Zhou Z, Miwa M, Hogetsu T (2001a) Polymorphism of simple sequence repeats reveals gene flow within and between ectomycorrhizal Suillus grevillei populations. New Phytol 149:339–348
Zhou Z, Miwa M, Matsuda Y, Hogetsu T (2001b) Spatial distribution of the subterranean mycelia and ectomycorrhizae of Suillus grevillei genets. J Plant Res 114:179–185
Acknowledgments
This study was partly supported by Grants-in-Aid (S) and (A) (No. 16101008 and 21248018) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Appendix. Excel macro program for an allele-clustering test
Appendix. Excel macro program for an allele-clustering test
The sheet used for the macro program was composed of three areas beneath a title row (R): data, allele-type and calculation ones. In the data area (column 2: C2 to C26), we placed genotypic data of each genet in a single row (R), which contained sequential cells for genet number (C2), sampling year (C3), patch number (C4), x-coordinate (C5), y-coordinate (C6) and paired alleles in all loci (C7 to C26). For Laccaria laccata data, C17 to C26 were blank.
In the allele-type area (C29 to C34), all allele types were manually picked from the genotype table of all genets (C7 to C26) and listed in C30 in order of locus, with their locus names in C29. The frequency and occurrence number of each allele type in a locus were calculated from the genotype table (C7 to C26) and entered in C31 and C32, in the row in which the allele type was listed. The numbers of all allele types listed in C30 (MAXALTYPE) and all genets in the data area (MAXGENET) were entered in R3C34 and R5C34, respectively.
In the calculation area (C36 to C41), cells in C36 and C37 were used by the program to output the actual average distance between positions of each allele type and the cluster score (CSCORE), respectively. When actual and simulated average distances between positions of each allele type were calculated, the program temporally stored the pair of x- and y-coordinates of all positions of the allele type in C38 and C39, respectively. The program also returned the combination of the number of each patch containing the allele type and its occurrence number in the patch, into cells to the right of C41. The macro program was written as follows:


Rights and permissions
About this article
Cite this article
Wadud, M.A., Nara, K., Lian, C. et al. Genet dynamics and ecological functions of the pioneer ectomycorrhizal fungi Laccaria amethystina and Laccaria laccata in a volcanic desert on Mount Fuji. Mycorrhiza 24, 551–563 (2014). https://doi.org/10.1007/s00572-014-0571-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-014-0571-x