Abstract
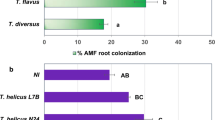
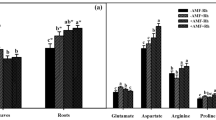
Plant growth-promoting rhizobacteria (PGPR) that produce antifungal metabolites are potential threats for the arbuscular mycorrhizal (AM) fungi known for their beneficial symbiosis with plants that is crucially important for low-input sustainable agriculture. To address this issue, we used a compartmented container system where test plants, Vigna radiata, could only reach a separate nutrient-rich compartment indirectly via the hyphae of AM fungi associated with their roots. In this system, where plants depended on nutrient uptake via AM symbiosis, we explored the impact of various PGPR. Plants were inoculated with or without a consortium of four species of AM fungi (Glomus coronatum, Glomus etunicatum, Glomus constrictum, and Glomus intraradices), and one or more of the following PGPR strains: phenazine producing (P+) and phenazine-less mutant (P−), diacetylphloroglucinol (DAPG) producing (G+) and DAPG-less mutant (G−) strains of Pseudomonas fluorescens, and an unknown antifungal metabolite-producing Alcaligenes faecalis strain, SLHRE425 (D). PGPR exerted only a small if any effect on the performance of AM symbiosis. G+ enhanced AM root colonization and had positive effects on shoot growth and nitrogen content when added alone, but not in combination with P+. D negatively influenced AM root colonization, but did not affect nutrient acquisition. Principal component analysis of all treatments indicated correlation between root weight, shoot weight, and nutrient uptake by AM fungus. The results indicate that antifungal metabolites producing PGPR do not necessarily interfere with AM symbiosis and may even promote it thus carefully chosen combinations of such bioinoculants could lead to better plant growth.







Similar content being viewed by others
References
Akköprü A, Demir S (2005) Biological control of Fusarium wilt in tomato caused by Fusarium oxysporum f. sp. lycopersici by AMF Glomus intraradices and some rhizobacteria. J Phytopathol 153:544–550. doi:10.1111/j.1439-0434.2005.01018.x
Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ Microbiol 8:1–10. doi:10.1111/j.1462-2920.2005.00942.x
Azcón R, Barea JM, Hayman DS (1976) Utilization of rock phosphate in alkaline soils by plants inoculated with mycorrhizal fungi and phosphate solubilizing bacteria. Soil Biol Biochem 8:135–138. doi:10.1016/0038-0717(76)90078-X
Azcón-Aguillar C, Barea JM (1995) Saprophytic growth of arbuscular–mycorrhizal fungi and other rhizospheric microorganisms. In: Allen MJ (ed) Mycorrhizal functioning: an integrative plant fungal process. Chapman and Hall, New York, N.Y, pp 163–198
Barea JM, Jeffries P (1995) Arbuscular mycorrhiza in sustainable soil plant systems. In: Hock B, Varma A (eds) Mycorrhiza structure, function, molecular biology and biotechnology. Springer, Heidelberg, pp 521–559
Barea JM, Andrade G, Bianciotto D, Lohrke S, Bonfante P, O`Gara F, Azcon-Aguillar C (1998) Impact on arbuscular mycorrhiza formation of Pseudomonas strains used as inoculants for biocontrol of soil-borne fungal plant pathogens. Appl Environ Microbiol 64:2304–2307
Barea JM, Azcon R, Azcon-Aguilar C (2002) Mycorrhizosphere interactions to improve plant fitness and soil quality. Anton Leeuw Int J G 81:343–351. doi:10.1023/A:1020588701325
Boronin AM, Thomashow LS (1998) A seven gene locus for synthesis of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2–79. J Bacteriol 180:2541–2548
Bowen GD, Theodorou C (1979) Interactions between bacteria and ectomycorrhizal fungi. Soil Biol Biochem 11:119–126. doi:10.1016/0038-0717(79)90087-7
Budi SW, van Tuinen D, Martinotti G, Gianinazzi S (1999) Isolation from the Sorghum bicolor mycorrhizosphere of a bacterium compatible with arbuscular mycorrhiza development and antagonistic towards soilborne fungal pathogens. Appl Environ Microbiol 65:5148–5150
Bull CT, Weller DM, Thomashow LS (1991) Relationship between root colonization and suppression of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens strain 2–79. Phytopathology 81:954–959. doi:10.1094/Phyto-81-954
Duponnois R, Plenchett C (2003) A mycorrhizal bacterium enhances ectomycorrhizal and endomycorrhizal symbiosis of Australian Acacia species. Mycorrhiza 13:85–91
Dwivedi D, Johri BN (2003) Antifungals from fluorescent pseudomonads: biosynthesis and regulation. Curr Sci 85:1693–1703
Ebel RC, Welbaum GE, Gunatilaka M, Nelson T, Auge RM (1996) Arbuscular mycorrhizal symbiosis and nonhydraulic signalling of soil drying in Vigna unguiculata (L.) Walp. Mycorrhiza 6:119–127. doi:10.1007/s005720050116
Frey-Klett P, Pieratt JC, Garbaye J (1997) Location and survival of mycorrhiza helper Pseudomonas fluorescens during establishment of ectomycorrhizal symbiosis between Laccaria bicolor and Douglas fir. Appl Environ Microbiol 63:139–144
Gamalero E, Trotta A, Massa N, Copetta A, Martinotti MG, Berta G (2004) Impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza 14:185–192. doi:10.1007/s00572-003-0256-3
Garbaye J (1994) Helper bacteria: a new dimension to the mycorrhizal symbiosis. New Phytol 128:197–210. doi:10.1111/j.1469-8137.1994.tb04003.x
Garland JL, Mill AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community level sole carbon source utilization. Appl Environ Microbiol 57:2351–2359
Gaur R, Shani N, Jeet K, Johri BN, Rossi P, Aragno M (2004) Diacetylphloroglucinol-producing pseudomonads do not influence AM fungi in wheat rhizosphere. Curr Sci 86:433–457
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular–arbuscular mycorrhizal infection in roots. New Phytol 124:221–230
Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823. doi:10.1038/nature03610
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319. doi:10.1038/nrmicro1129
Haas D, Keel C (2003) Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant diseases. Annu Rev Phytopathol 41:117–153. doi:10.1146/annurev.phyto.41.052002.095656
Harrison MJ (2005) Signalling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59:19–42. doi:10.1146/annurev.micro.58.030603.123749
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular 347. Agricultural Experiment Station, University of California, Berkeley
Hodge A (2001) Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient plant capture from, glycine patches in soil. New Phytol 151:725–734. doi:10.1046/j.0028-646x.2001.00200.x
Ianson DC, Linderman RG (1993) Variation in the response of nodulating pigeonpea (Cajanus cajan) to different isolates of mycorrhizal fungi. Symbiosis 15:105–119
Johnson D, Leake JR, Ostle N, Ineson P, Read DJ (2002) In situ (CO2)-C-13 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol 153:327–334. doi:10.1046/j.0028-646X.2001.00316.x
Keel C, Schnider U, Maurhofer M, Voisard C, Burger D, Haas D, Défago G (1992) Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2, 4-diacetylphloroglucinol. Mol Plant Microbe Interact 5:4–13
Kloepper JW (1993) Plant growth promoting rhizobacteria as biological control agents. In: Metting FB Jr (ed) Soil microbia ecology—applications in agricultural and environmental management. Marcel Dekker, New York, pp 255–274
Landa BB, Mavrodi OV, Raaijmakers JM, Gardener BBM, Thomashow LS, Weller DM (2002) Differential ability of genotypes of 2, 4-diacetylphloroglucinol-producing Pseudomonas fluorescens strains to colonize the roots of pea plants. Appl Environ Microbiol 68:3226–3237. doi:10.1128/AEM.68.7.3226-3237.2002
Langmeier M, Oberson A, Kreuzer M, Frossard E (1998) N fertilizer efficiency of cattle manure. Part I: 15N labeling of cow excreta. First Symposium of the Competence Centre for Plant Sciences, 10 Dec, Zurich
Legendre P, Legendre L (1998) Numerical ecology. Second English edition. Elsevier, Amsterdam
Levy A, Chang BJ, Abbott LK, Kuo J, Harnett G, Inglis TJJ (2003) Invasion of spores of the arbuscular mycorrhizal fungus Gigaspora decipiens by Burkholderia spp. Appl Environ Microbiol 69:6250–6256. doi:10.1128/AEM.69.10.6250-6256.2003
Linderman RG (1988) Mycorrhizal interactions with the rhizosphere microflora: the mycorrhizospheric effect. Phytopathology 78:366–371
Linderman RG (2000) Effects of mycorrhizas on plant tolerance to disease. In: Kapulnik Y, Douds DD (eds) Arbuscular mycorrhizas: physiology of function. Kluwer, Dordrecht, pp 345–365
Mäder P, Vierheilig H, Streitwolf-Engel R, Boller T, Frey B, Christie P, Wiemken A (2000) Transport of 15N from a soil compartment separated by a poly tetrafluoroethylene membrane to plant roots via the hyphae of arbuscular mycorrhizal fungi. New Phytol 146:155–161. doi:10.1046/j.1469-8137.2000.00615.x
Meyer JR, Linderman RG (1986a) Response of subterranean clover to dual inoculation with vesicular–arbuscular mycorrhizal fungi and a plant growth-promoting bacterium, Pseudomonas putida. Soil Biol Biochem 18:185–190. doi:10.1016/0038-0717(86)90025-8
Meyer JR, Linderman RG (1986b) Selective influence on populations of rhizosphere and rhizoplane bacteria and actinomycetes by mycorrhiza formed by Glomus fasciculatum. Soil Biol Biochem 18:191–196. doi:10.1016/0038-0717(86)90026-X
Oehl F, Sieverding E, Ineichen K, Mäder P, Boller T, Wiemken A (2003) Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of central Europe. Appl Environ Microbiol 69:2816–2824. doi:10.1128/AEM.69.5.2816-2824.2003
Onishi T, Gall RS, Mayer ML (1975) An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem 69:261–267. doi:10.1016/0003-2697(75)90585-0
Paulitz TC, Linderman RG (1989) Interactions between fluorescent pseudomonads and VA mycorrhizal fungi. New Phytol 113:37–45. doi:10.1111/j.1469-8137.1989.tb02393.x
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158
Pielou EC (1984) The interpretation of ecological data: a data on classification and ordination. Wiley, New York
Raaijmakers JM, Weller DM (1998) Natural plant protection by 2,4-diacetyl-phloroglucinol-producing Pseudomonas spp. in take-all decline soils. Mol Plant Microbe Interact 11:144–152. doi:10.1094/MPMI.1998.11.2.144
Raaijmakers JM, Bonsall RF, Weller DM (1999) Effect of population density of Pseudomonas fluorescens on production of 2, 4-diacetylphloroglucinol in the rhizosphere of wheat. Phytopathology 89:470–475. doi:10.1094/PHYTO.1999.89.6.470
Raaijmakers JM, de Bruijn I, de Kock MJD (2006) Cyclic lipopeptide production by plant-associated Pseudomonas spp.: diversity, activity, biosynthesis, and regulation. Mol Plant Microbe Interact 19:699–710. doi:10.1094/MPMI-19-0699
Rainey PB (1999) Adaptation of Pseudomonas fluorescens to the plant rhizosphere. Appl Environ Microbiol 1:243–257. doi:10.1046/j.1462-2920.1999.00040.x
Ravnskov S, Nybore O, Jakobsen I (1999) Influence of an arbuscular mycorrhizal fungus on Pseudomonas fluorescens DF57 in rhizosphere and hyphosphere soil. New Phytol 142:113–122. doi:10.1046/j.1469-8137.1999.00374.x
Rezzonico F, Zala M, Keel C, Duffy C, Moënne-Loccoz Y, Défago G (2007) Is the ability of biocontrol fluorescent pseudomonads to produce the antifungal metabolite 2, 4-diacetylphloroglucinol really synonymous with higher plant protection? New Phytol 173:861–872. doi:10.1111/j.1469-8137.2006.01955.x
Rhodes LH, Gerdemann JW (1975) Phosphate uptake zones of mycorrhizal and non-mycorrhizal onions. New Phytol 75:555–561. doi:10.1111/j.1469-8137.1975.tb01419.x
Schnider-Keel U, Keel C, Blumer C, Troxer J, Défago G, Haas D (1995) Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J Bacteriol 177:5387–5392
Schreiner RP, Mihara KL, McDaniel H, Bethlenfalvay GJ (1997) Mycorrhizal fungi influence plant and soil functions and interactions. Plant Soil 188:199–209. doi:10.1023/A:1004271525014
Schussler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421. doi:10.1017/S0953756201005196
Singh S, Kapoor KK (1998) Effects of inoculation of phosphate solubilizing microorganisms and an arbuscular mycorrhizal fungus on mungbean grown under natural soil conditions. Mycorrhiza 7:249–253. doi:10.1007/s005720050188
Smith FA, Read SE (1997) Structural diversity in (vesicular)–arbuscular mycorrhizal symbiosis. New Phytol 137:373–388. doi:10.1046/j.1469-8137.1997.00848.x
Thomashow LS, Weller DM (1995) Plant microbe interact vol. 1. In: Stacey G, Keen N (eds) Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites. Chapman and Hall, New York, pp 187–235
Toljander JF, Artursson V, Paul LR, Janssom JK, Finlay RD (2006) Attachment of different soil bacteria to arbuscular mycorrhizal fungal extraradical hyphae is determined by hyphal vitality and fungal species. FEMS Microbiol Lett 254:34–40. doi:10.1111/j.1574-6968.2005.00003.x
Toro M, Azcon R, Barea JM (1997) Improvement of arbuscular mycorrhiza development by inoculation of soil with phosphate solubilizing rhizobacteria to improve rock phosphate bioavailability (P-32) and nutrient cycling. Appl Environ Microbiol 63:4408–4412
Vázquez MM, César S, Azcón R, Barea JM (2000) Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl Soil Ecol 15:261–272. doi:10.1016/S0929-1393(00)00075-5
Vosátka M, Gryndler M (1999) Treatment with culture fractions from Pseudomonas putida modifies the development of Glomus fistulosum mycorrhiza and the response of potato and maize plants to inoculation. Appl Soil Ecol 11:245–251. doi:10.1016/S0929-1393(98)00151-6
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sc Soc Am Proc 29:677–678
Weller DM, Landa BB, Mavrodi OV, Schroeder KL, De La Fuente L, Bankhead SB, Molar RA, Bonsall RF, Mavrodi DV, Thomashow LS (2007) Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol 9:4–20. doi:10.1055/s-2006-924473
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Wyss P, Boller T, Wiemken A (1991) Phytoalexin response is elicited by a pathogen Rhizoctonia solani but not by mycorrhizal fungus (Glomus mosseae) in soybean roots. Experientia 47:395–399. doi:10.1007/BF01972082
Xavier LJC, Germida JJ (2003) Bacteria associated with Glomus clarum spores influence mycorrhizal activity. Soil Biol Biochem 35:471–478. doi:10.1016/S0038-0717(03)00003-8
Acknowledgments
The authors would like to thank Alok Adholeya for providing some mycorrhizal inocula; Geneviéve Défago and Linda Thomashow for providing the Pseudomonas cultures; and David Roesti, Feng He, and Bharani Kumar for statistical analysis and interpretation. We thank Roel M. Schaaper, Rajesh Kasiviswanathan, and Greg Stuart for critical comments on the manuscript. This work was funded by Indo-Swiss Collaboration in Biotechnology (ISCB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dwivedi, D., Johri, B.N., Ineichen, K. et al. Impact of antifungals producing rhizobacteria on the performance of Vigna radiata in the presence of arbuscular mycorrhizal fungi. Mycorrhiza 19, 559–570 (2009). https://doi.org/10.1007/s00572-009-0253-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-009-0253-2