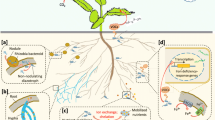
Abstract
Roots undergo multiple changes as a consequence of arbuscular mycorrhizal (AM) interactions. One of the major alterations expected is the induction of membrane transport systems, including proton pumps. In this work, we investigated the changes in the activities of vacuolar and plasma membrane (PM) H+ pumps from maize roots (Zea mays L.) in response to colonization by two species of AM fungi, Gigaspora margarita and Glomus clarum. Both the vacuolar and PM H+-ATPase activities were inhibited, while a concomitant strong stimulation of the vacuolar H+-PPase was found in the early stages of root colonization by G. clarum (30 days after inoculation), localized in the younger root regions. In contrast, roots colonized by G. margarita exhibited only stimulation of these enzymatic activities, suggesting a species-specific phenomenon. However, when the root surface H+ effluxes were recorded using a noninvasive vibrating probe technique, a striking activation of the PM H+-ATPases was revealed specifically in the elongation zone of roots colonized with G. clarum. The data provide evidences for a coordinated regulation of the H+ pumps, which depicts a mechanism underlying an activation of the root H+-PPase activity as an adaptative response to the energetic changes faced by the host root during the early stages of the AM interaction.






Similar content being viewed by others
References
Bago B, Donaire JP, Azcón-Aguilar C (1997) ATPases activities of root from mycorrhizal sunflower (Helianthus annuus) and onion (Allium cepa) plants. New Phytol 136:305–311. doi:10.1046/j.1469-8137.1997.00741.x
Bago B, Azcon-Aguilar C, Piché Y (1998) Architecture and developmental dynamics of the external mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown under monoxenic conditions. Mycologia 90:52–62. doi:10.2307/3761011
Bécard G, Fortin J (1988) Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol 108:211–218. doi:10.1111/j.1469-8137.1988.tb03698.x
Benabdellah K, Azcón-Aguilar C, Ferrol N (1999) Plasma membrane ATPase and H+ transport activities in microsomal membranes from mycorrhizal tomato roots. J Exp Bot 50:1343–1349. doi:10.1093/jexbot/50.337.1343
Berta G, Fusconi A, Trotta A (1993) VA mycorrhizal infection and the morphology and function of root systems. Environ Exp Bot 33:159–173. doi:10.1016/0098-8472(93)90063-L
Bestel-Corre G, Dumas-Gaudot E, Poinsot V, Dieu M, Dierick JF, van Tuinen D, Remacle J, Gianinazzi-Pearson V, Gianinazzi S (2002) Proteome analysis and identification of symbiosis-related proteins from Medicago truncatula Gaertn. by two-dimensional electrophoresis and mass spectrometry. Electrophoresis 23:122–137
Bonfante-Fasolo P, Perotto S (1992) Plant and endomycorrhizal fungi: the cellular and molecular basis of their interaction. In: Verma DPS (ed) Molecular signals in plant-microbe communications. CRC, Boca Raton, FL, pp 445–470
Buch-Pedersen MJ, Rudashevskaya EL, Berner TS, Venema K, Palmgren MG (2006) Potassium as an intrinsic uncoupler of the plasma membrane H+-ATPase. J Biol Chem 281:38285–38292. doi:10.1074/jbc.M604781200
Cappellazzo G, Lanfranco L, Fitz M, Wipf D, Bonfante P (2008) Characterization of an amino acid permease from the Endomycorrhizal fungus Glomus mosseae. Plant Physiol 147:429–437. doi:10.1104/pp.108.117820
Carystinos GD, MacDonald HR, Monroy AF, Dhindsa RS, Poole RJ (1995) Vacuolar H+-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiol 108:641–649. doi:10.1104/pp.108.2.641
Clark RB (1975) Characterization of phosphatase of intact maize roots. J Agric Food Chem 23:458–460. doi:10.1021/jf60199a002
Cruz C, Egsgaard H, Trujillo C, Ambus P, Requena N, Martins-Loução MA, Jakobsen I (2007) Enzymatic evidence for the key role of arginine in nitrogen translocation by arbuscular mycorrhizal fungi. Plant Physiol 144:782–792. doi:10.1104/pp.106.090522
Davies JM, Darley CP, Sanders D (1997) Energetics of the plasma membrane pyrophosphatase. Trends Plant Sci 2:9–10. doi:10.1016/S1360-1385(97)82732-X
De Michelis MI, Spanswick RM (1986) H+-pumping driven by vanadate sensitive ATPase in membrane vesicles from corns roots. Plant Physiol 81:542–547
Drozdowicz YM, Rea PA (2001) Vacuolar H+-pyrophosphatases: from evolutionary backwaters into mainstream. Trends Plant Sci 6:206–211. doi:10.1016/S1360-1385(01)01923-9
Façanha AR, De Meis L (1995) Inhibition of maize root H+-ATPase by fluoride and fluoroaluminate complexes. Plant Physiol 108:241–246
Façanha AR, De Meis L (1998) Reversibility of H+-ATPase and H+-Pyrophosphatase in tonoplast vesicles from maize coleoptiles and seeds. Plant Physiol 116:1487–1495. doi:10.1104/pp.116.4.1487
Feijó JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK (1999) Growing pollen tubes posses a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J Cell Biol 144:483–496. doi:10.1083/jcb.144.3.483
Ferrol N, Pozo MJ, Antelo M, Azcón-Aguilar C (2002) Arbuscular mycorrhizal symbiosis regulates plasma membrane H+-ATPase gene expression in tomato plants. J Exp Bot 53:1683–1687. doi:10.1093/jxb/erf014
Fieschi M, Alloatti G, Sacco S, Berta G (1992) Membrane-potential hyperpolarization in vesicular arbuscular mycorrhizae of Allium porrum L.: A non-nutritional long-distance effect of the fungus. Protoplasma 168:136–140. doi:10.1007/BF01666259
Fiske CF, Subbarow Y (1925) The colorometric determination of phosphorus. J Biol Chem 66:375–400
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214. doi:10.1016/j.febslet.2007.03.050
Gianinazzi S (1991) Vesicular-arbuscular (endo-) mycorrhizas: cellular, biochemical and genetic aspects. Agric Ecosyst Environ 35:105–119
Gianinazzi-Pearson V (1996) Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. Plant Cell 8:1871–1883
Gianinazzi-Pearson V, Brechenmacher L (2004) Functional genomics of arbuscular mycorrhiza: decoding the symbiotic cell programme. Can J Bot 82:1228–1234. doi:10.1139/b04-096
Gianinazzi-Pearson V, Smith SE, Gianinazzi S, Smith FA (1991) Enzymatic studies on the metabolism of vesicular–arbuscular mycorrhiza. V. Is H+-ATPase a component of ATP-Hydrolysing enzyme activities in plant-fungi interfaces? New Phytol 117:61–74. doi:10.1111/j.1469-8137.1991.tb00945.x
Gianinazzi-Pearson V, Arnould C, Oufattole M, Arango M, Gianinazzi S (2000) Differential activation of H+-ATPase genes by an arbuscular mycorrhizal fungus in root cells of transgenic tobacco. Planta 211:609–613. doi:10.1007/s004250000323
Giovannetti M, Mosse B (1980) An evaluation of techniques to measure vesicular–arbuscular mycorrhizal infection in roots. New Phytol 84:484–500. doi:10.1111/j.1469-8137.1980.tb04556.x
Giovannetti M, Sbrana C, Avio L, Citernesi AS, Logi C (1993) Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during preinfection stages. New Phytol 125:587–593. doi:10.1111/j.1469-8137.1993.tb03907.x
Giovannetti M, Sbrana C, Citernesi AS, Avio L (1996) Analysis of factors involved in fungal recognition responses to host derived signals by arbuscular mycorrhizal fungi. New Phytol 133:65–71. doi:10.1111/j.1469-8137.1996.tb04342.x
Graham JH, Eissenstat DM (1994) Host genotype and the formation and function of VA mycorrhizae. Plant Soil 159:179–185
Guttenberger M (2000) Arbuscules of vesicular–arbuscular mycorrhizal fungi inhabit an acidic compartment within plant roots. Planta 211:112–118. doi:10.1007/s004250000324
Harrison MJ (1996) A sugar transporter from Medicago truncatula: altered expression pattern in roots during vesicular–arbuscular (VA) mycorrhizal associations. Plant J 9:491–503. doi:10.1046/j.1365-313X.1996.09040491.x
Harrison MJ (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59:19–42. doi:10.1146/annurev.micro.58.030603.123749
Hohnjec N, Vieweg MF, Pühler A, Becker A, Küster H (2005) Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiol 137:1283–1301. doi:10.1104/pp.104.056572
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Statist 5:299–314. doi:10.2307/1390807
Jahn T, Baluska F, Michalke W, Harper JF, Volkmann D (1998) Plasma membrane H+-ATPase in the root apex: evidence for strong expression in xylem parenchyma and asymmetric localization within cortical and epidermal cells. Physiol Plant 104:311–316. doi:10.1034/j.1399-3054.1998.1040304.x
James EK, Reis VM, Olivares FL, Baldani J, Döbereiner J (1994) Infection of sugar cane by the nitrogen-fixing bacterium Acetobacter diazotrophicus. J Exp Bot 45:757–766. doi:10.1093/jxb/45.6.757
Krajinski F, Hause B, Gianinazzi-Pearson V, Franken P (2002) Mtha1, a plasma membrane H+-ATPase gene from Medicago truncatula, shows arbuscule-specific induced expression in mycorrhizal tissue. Plant Biol 4:754–761. doi:10.1055/s-2002-37407
Kunkel JG, Cordeiro S, Xu J, Shipley AM, Feijó JA (2006) The use of non-invasive ion-selective microelectrode techniques for the study of plant development. In: Volkov V (ed) Plant electrophysiology—theory and methods. Springer, Berlin, pp 109–137
Lambais MR (2006) Unraveling the signaling and signal transduction mechanisms controlling arbuscular mycorrhiza development. Scientia Agricola 63:405–413. doi:10.1590/S0103-90162006000400013
Li H, Smith FA, Dickson S, Holloway RE, Smith SE (2008) Plant growth depressions in arbuscular mycorrhizal symbioses: not just caused by carbon drain? New Phytol 178:852–862. doi:10.1111/j.1469-8137.2008.02410.x
Lichko L, Okorokov L (1991) Purification and some properties of membrane bound and soluble pyrophosphatase of yeast vacuoles. Yeast 7:805–812. doi:10.1002/yea.320070805
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marx C, Dexheimer J, Gianinazzi-Pearson V, Gianinazzi S (1982) Enzymatic studies on the metabolism of vesicular–arbuscular mycorrhiza. IV. Ultracitoenzymological evidence (ATPase) for active transfer processes in the host-arbuscular interface. New Phytol 90:37–43. doi:10.1111/j.1469-8137.1982.tb03238.x
Mcarthur DAJ, Knowles NR (1993) Influence of vesicular–arbuscular mycorrhizal fungi on the response of potato to phosphorus deficiency. Plant Physiol 101:147–160
Mito N, Wimmers LE, Bennett AB (1996) Sugar regulates mRNA abundance of H+-ATPase gene family members in tomato. Plant Physiol 112:1229–1236. doi:10.1104/pp.112.3.1229
Morsomme P, Boutry M (2000) The plant plasma membrane H+-ATPase: structure, function and regulation. Biochim Biophys Acta 1465:1–16. doi:10.1016/S0005-2736(00)00128-0
Murphy PJ, Langridge P, Smith SE (1996) Cloning plant genes differentially expressed during colonization of roots of Hordeum vulgare by the vesicular–arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 135:291–301. doi:10.1046/j.1469-8137.1997.00652.x
Nakanishi Y, Maeshima M (1998) Molecular cloning of vacuolar H+-pyrophosphatase and its developmental expression in growing hypocotyl of mung bean. Plant Physiol 116:589–597. doi:10.1104/pp.116.2.589
Olivares FL, James EK, Baldani JI, Döbereiner J (1997) Infection of mottled stripe disease susceptible and resistant varieties of sugar cane by the endophytic diazotroph Herbaspirillum. New Phytol 135:723–737. doi:10.1046/j.1469-8137.1997.00684.x
Okorokov LA, Silva FE, Okorokova-Façanha AL (2001) Ca2+ and H+ homeostasis in fission yeast: a role of Ca2+/H+ exchange and distinct V-H+-ATPases of the secretory pathway organelles. FEBS Lett 505:321–324. doi:10.1016/S0014-5793(01)02852-6
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845. doi:10.1146/annurev.arplant.52.1.817
Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA (2005) Up-regulation of a H+-pyrophosphatase (H+-PPase) as strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci U S A 102:18830–18835. doi:10.1073/pnas.0509512102
Philips JM, Hayman DS (1970) Improved procedure for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Portillo F (2000) Regulation of plasma membrane H+-ATPase in fungi and plants. Biochim Et Biophys Acta-Rev Biomembr 1469:31–42
Poulsen KH, Nagy R, Gao LL, Smith SE, Bucher M, Smith FA, Jakobsen I (2005) Physiological and molecular evidence for pi uptake via the symbiotic pathway in a reduced mycorrhizal colonization mutant in tomato associated with a compatible fungus. New Phytol 168:445–454. doi:10.1111/j.1469-8137.2005.01523.x
Ramos AC, Martins MA, Façanha AR (2005) ATPase and pyrophosphatase activities in corn root microsomes colonized with arbuscular mycorrhizal fungi. Braz J Soil Sci 29:207–213
Ramos AC, Façanha AR, Feijó JA (2008a) Ion dynamics during the polarized growth of arbuscular mycorrhizal fungi: from presymbiosis to symbiosis. In: Varma A, Hock B (eds) Mycorrhiza. Springer, Germany, pp 241–261
Ramos AC, Façanha AR, Feijó JA (2008b) A proton (H+) flux signature of the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol 178:177–188. doi:10.1111/j.1469-8137.2007.02344.x
Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 99:1271–1274
Rausch C, Daram P, Brunner S, Jansa J, Laloi M, Leggewie G, Amrhein N, Bucher M (2001) A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 414:462–466. doi:10.1038/35106601
Rosewarne GM, Smith FA, Schachtman DP, Smith SE (2007) Localization of proton-ATPase genes expressed in arbuscular mycorrhizal tomato plants. Mycorrhiza 17:249–258. doi:10.1007/s00572-006-0101-6
Schaller A, Oecking C (1999) Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 11:263–272
Schüβler A, Martin H, Cohen D, Fitz M, Wipf D (2006) Characterization of a carbohydrate transporter from symbiotic glomeromycotan fungi. Nature 444:933–936
Siciliano V, Genre A, Balestrini R, Cappellazzo G, deWit PJGM, Bonfante P (2007) Transcriptome analysis of arbuscular mycorrhizal roots during development of the prepenetration apparatus. Plant Physiol 144:1455–1466. doi:10.1104/pp.107.097980
Smith SE, Smith FA (1990) Structure and function of the interfaces in biotrophic symbioses as they relate to nutrient transport. New Phytol 144:1–38
Sondergaard TE, Schulz A, Palmgren MG (2004) Energization of transport processes in plants. roles of the plasma membrane H+-ATPase. Plant Physiol 136:2475–2482. doi:10.1104/pp.104.048231
Valot B, Dieu M, Recorbet G, Raes M, Gianinazzi S, Dumas-Gaudot E (2005) Identification of membrane-associated proteins regulated by the arbuscular mycorrhizal symbiosis. Plant Mol Biol 59:565–580
Willians K, Percival F, Merino J, Mooney HA (1987) Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant Cell Environ 10:725–734
Wright DP, Read DJ, Scholes JD (1998a) Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ 21:881–891. doi:10.1046/j.1365-3040.1998.00351.x
Wright DP, Scholes JD, Read DJ (1998b) Effects of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant Cell Environ 21:209–216. doi:10.1046/j.1365-3040.1998.00280.x
Xing T, Higgins VJ, Blumwald E (1996) Regulation of plant defense response to fungal pathogens: two types of protein kinases in the reversible phosphorylation of the host plasma membrane H+-ATPase. Plant Cell 8:555–564
Zandonadi DB, Canellas LP, Facanha AR (2007) Indolacetic and humic acids induce lateral root development through a concentrated plasmalemma and tonoplast H+-pumps activation. Planta 225:1583–1595. doi:10.1007/s00425-006-0454-2
Acknowledgments
This work was supported by CAPES (Brazil) and by Post-Doctoral fellowship (SFRH/BPD/21061/2004) conceded to ACR by Fundação para a Ciência e Tecnologia and Instituto Gulbenkian de Ciência (Portugal). ARF is supported by grants from International Foundation for Science (IFS) (C/3483-1) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (475522/01-0 and 479286/03-5). The authors would like to acknowledge Dr. Carlos Tadokoro and Dr Rui Gardner for the manuscript revision, and Dr. Mark Seldon for the critical review and helpful suggestions of the manuscript. We also thank the Microscopy Center “Raul Dodsworth Machado” (UENF-BRAZIL) and Quíssila Batista for the help in preparing the samples for microscopy analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM
a Light microscopy (LM) of cross-section of maize roots var UENF 506-6 colonized by G. clarum at 30 days after inoculation. Note the presence of intraradical hyphae mainly at the outer cortex colonizing intercellular parenchymatic cells (arrowhead) and penetrating through the cortical cells (arrow). Note that hyphae-infected cells are hypertrophic. cc cortical cell, magnification 750×. b Transmission electron microscopy (TEM) showing intraradical hyphae of G. Clarum penetrating parenchymatic cortical cell (arrow) during the earlier steps of the mycorrhizal interaction with maize roots. ld lipid droplets, ie intercellular space, cc cortical cells, magnification 3,000×. c LM of cross-section of maize roots var. UENF 506-6 colonized by G. Clarum at more advanced stage of the symbiotic establishment. Note a significant proportion of colonization of the cortical tissue by VAM structures including arbuscules, magnification 200×. d TEM from previous photomicrograph showing a portion of an arbuscule and intracellular hyphae into a hypertrophic cortical cell (star) (GIF 150 KB)
Rights and permissions
About this article
Cite this article
Ramos, A.C., Martins, M.A., Okorokova-Façanha, A.L. et al. Arbuscular mycorrhizal fungi induce differential activation of the plasma membrane and vacuolar H+ pumps in maize roots. Mycorrhiza 19, 69–80 (2009). https://doi.org/10.1007/s00572-008-0204-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-008-0204-3