Abstract
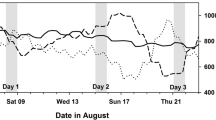
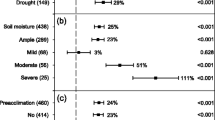
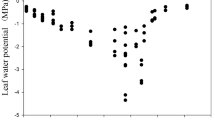
Stomatal conductance (g s) and transpiration rates vary widely across plant species. Leaf hydraulic conductance (k leaf) tends to change with g s, to maintain hydraulic homeostasis and prevent wide and potentially harmful fluctuations in transpiration-induced water potential gradients across the leaf (ΔΨ leaf). Because arbuscular mycorrhizal (AM) symbiosis often increases g s in the plant host, we tested whether the symbiosis affects leaf hydraulic homeostasis. Specifically, we tested whether k leaf changes with g s to maintain ΔΨ leaf or whether ΔΨ leaf differs when g s differs in AM and non-AM plants. Colonization of squash plants with Glomus intraradices resulted in increased g s relative to non-AM controls, by an average of 27% under amply watered, unstressed conditions. Stomatal conductance was similar in AM and non-AM plants with exposure to NaCl stress. Across all AM and NaCl treatments, k leaf did change in synchrony with g s (positive correlation of g s and k leaf), corroborating leaf tendency toward hydraulic homeostasis under varying rates of transpirational water loss. However, k leaf did not increase in AM plants to compensate for the higher g s of unstressed AM plants relative to non-AM plants. Consequently, ΔΨ leaf did tend to be higher in AM leaves. A trend toward slightly higher ΔΨ leaf has been observed recently in more highly evolved plant taxa having higher productivity. Higher ΔΨ leaf in leaves of mycorrhizal plants would therefore be consistent with the higher rates of gas exchange that often accompany mycorrhizal symbiosis and that are presumed to be necessary to supply the carbon needs of the fungal symbiont.


Similar content being viewed by others
Abbreviations
- AM:
-
arbuscular mycorrhizal
- k leaf :
-
leaf hydraulic conductance
- g s :
-
stomatal conductance
- ΔΨ leaf :
-
transpiration-induced water potential gradient, or drawdown, across the leaf
References
Allen MF (1982) Influence of vesicular-arbuscular mycorrhizae on water movement through Bouteloua gracilis (H.B.K.) Lag ex Steud. New Phytol 91:191–196
Allen MF, Smith WK, Moore TS Jr, Christensen M (1981) Comparative water relations and photosynthesis of mycorrhizal and non-mycorrhizal Bouteloua gracilis H.B.K. New Phytol 88:683–693
Augé RM (2001) Water relations, drought and VA mycorrhizal symbiosis. Mycorrhiza 11:3–42
Augé RM (2000) Stomatal behavior of arbuscular mycorrhizal plants. In: Kapulnik Y, Douds DD (eds) Arbuscular mycorrhizas: physiology and function. Kluwer, Dordrecht, The Netherlands, pp 201–237
Augé RM, Moore JL, Cho K, Stutz JC, Sylvia DM, Al-Agely AK, Saxton AM (2003) Relating foliar dehydration tolerance of mycorrhizal Phaseolus vulgaris to soil and root colonization by hyphae. J Plant Physiol 160:1147–1156
Augé RM, Moore JL, Sylvia DM, Cho K (2004a) Mycorrhizal promotion of host stomatal conductance in relation to irradiance and temperature. Mycorrhiza 14:85–92
Augé RM, Stodola AJW, Tims JE, Saxton AM (2001) Moisture retention properties of a mycorrhizal soil. Plant Soil 230:87–97
Augé RM, Sylvia DM, Park SJ, Buttery BR, Saxton AM, Moore JL, Cho K (2004b) Partitioning mycorrhizal influence on water relations of Phaseolus vulgaris into soil and plant components. Can J Bot 82:503–514
Augé RM, Toler HD, Moore JL, Cho K, Saxton AM (2007) Comparing contributions of soil versus root colonization to variations in stomatal behavior and soil drying in mycorrhizal Sorghum bicolor and Cucurbita pepo. J Plant Physiol 164:1289–1299
Bryla DR, Duniway JM (1997) Growth, phosphorus uptake, and water relations of safflower and wheat infected with an arbuscular mycorrhizal fungus. New Phytol 136:581–590
Cho K, Toler HD, Lee J, Ownley BH, Jean C Stutz JC, Moore JL, Augé RM (2006) Mycorrhizal symbiosis and response of sorghum plants to combined drought and salinity stresses. J Plant Physiol 163:517–528
Cowan IR, Farquhar GD (1997) Stomatal function in relation to leaf metabolism and environment. In: Jennings DH (ed) Integration of activity in the higher plant. Cambridge University Press, Cambridge, UK, pp 471–505
Farquhar GD, Buckley TN, Miller JM (2002) Optimal stomatal control in relation to leaf area and nitrogen content. Silva Fenn 36:625–637
Franks P (2006) Higher rates of leaf gas exchange are associated with higher leaf hydrodynamic pressure gradients. Plant Cell Environ 29:584–592
Khalvati MA, Hu Y, Mozafar A, Schmidhalter U (2005) Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biol 7:706–712
Mansfield TA, Hetherington AM, Atkinson CJ (1990) Some current aspects of stomatal physiology. Annu Rev Plant Physiol Plant Mol Biol 41:55–75
Radin JW (1984) Stomatal responses to water stress and to abscisic acid in phosphorus-deficient cotton plants. Plant Physiol 76:392–394
Oparka KJ, Prior DAM (1992) Direct evidence of pressure-generated closure of plasmodesmata. Plant Journal 2:741–750
Querejeta JI, Allen MF, Caravaca F, Roldan A (2006) Differential modulation of host plant delta C-13 and delta O-18 by native and nonnative arbuscular mycorrhizal fungi in a semiarid environment. New Phytol 169:379–387
Ruiz-Lozano JM, Azcón R (1997) Effect of calcium application on the tolerance of mycorrhizal lettuce plants to polyethylene glycol-induced water stress. Symbiosis 23:9–21
Ruiz-Lozano JM, Azcón R, Gomez M (1996) Alleviation of salt stress by arbuscular-mycorrhizal Glomus species in Lactuca sativa plants. Physiol Plant 98:767–772
Schulte PJ (1993) Tissue properties and water relations of desert shrubs. In: Smith JAC, Griffiths H (eds) Water deficits: plant responses from cell to community. Bios Scientific, Oxford, UK, pp 177–192
Taiz L, Zeiger E (2006) Plant physiology, 4th edn. Sinauer, Sunderland, MA
Tyree MT, Cheung YNS (1977) Resistance to water flow in Fagus grandifolia leaves. Can J Bot 55:2591–2599
Acknowledgments
This work was supported by the Agricultural Experiment Station at the University of Tennessee. We are grateful to Casey Barrickman for nutrient analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Augé, R.M., Toler, H.D., Sams, C.E. et al. Hydraulic conductance and water potential gradients in squash leaves showing mycorrhiza-induced increases in stomatal conductance. Mycorrhiza 18, 115–121 (2008). https://doi.org/10.1007/s00572-008-0162-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-008-0162-9