Abstract
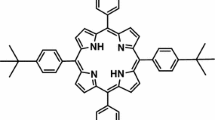
The presented work focuses on the investigations of a metallo-porphyrin and its gasochromic behavior to different gases. Gasochromic materials change their color while they are exposed to a certain gas. So they offer the possibility to develop highly selective chemical gas sensors and gas sensing systems. The focus of this work is the characterization of the metallo-porphyrin 5, 10, 15, 20-tetraphenylporphyrin-zinc (ZnTPP). Nonetheless, there is a wide range of other possible metallo-porphyrins. When embedded into a polymeric matrix (PVC) a color change to the toxic gas NO2 can be detected. To develop a stand-alone gas sensor, the porphyrin/PVC matrix is deposited onto a planar optical waveguide. The color change of the porphyrin dye can be detected in the evanescent field of the optical waveguide. Therefore, the light of a high power LED is coupled into the waveguide. The color change of the porphyrin is detectable with photodiodes as a variation of the out-coupled light intensity. The sensor shows no unwished sensitivities to CO2 and CO and only low to NH3. NO2 is detectable with a resolution of 1 ppm.









Similar content being viewed by others
References
Courbat J, Briand D, Damon-Lacoste J, Wöllenstein J, de Rooij NF (2009) Evaluation of pH indicator-based colorimetric films for ammonia detection using optical waveguides. Sens Actuators B 143:62–70
Dräger (2011) http://www.draeger.de/media/10/00/92/10009262/labor_hinter_glas_br_9046078_de.pdf, available at 15.03.2011
Filippini D (2006) Chemical sensing with familiar devices. Angew Chem Int Ed 45:3800–3803
Kurtikyan TS, Ford PC (2008) FTIR and optical spectroscopic studies of the reaction of heme models with nitric oxide and other NOx in porous layered solids. Coord Chem Rev 252:1486–1496
Kurtikyan TS, Stephanyan TG, Gasparayan AV, Zhamkochyan GA (1998) Interaction of nitrogen dioxide with sublimed films of meso-tetraphenylporphyrinatozinc. Russian Chem Bull 47:644–647
Lim MD, Lorkovic IM, Ford PC (2005) NO and NOx interactions with group 8 metalloporphyrins. J Inorg Biochem 99:151–165
Mansuy D (2007) A brief history of the contribution of metalloporphyrin models to cytochrome P450 chemistry and oxidation catalysis. Compt Rend Chim 10(4–5):392–413
Matsui T, Unno M, Ikeda-Saito M (2010) Heme oxygenase reveals its strategy for catalyzing three successive oxygenation reactions. Acc Chem Res 43(2):240–247
Nakano N (1996) Development of a monitoring tape for nitrogen dioxide in air. Anal Chim Acta 321:41–45
Nezel T, Spichiger-Keller UE, Ludin C, Hensel A (2001) Gas-selective optical sensors for fire detectors. CHIMIA Int J Chem 55:725–731
Saltzman BE (1954) Colorimetric microdetermination of nitrogen dioxide in the atmosphere. Anal Chem 26:1949–1955
Shine HJ, Padilla AG, Wu SM (1979) Reactions of zinc tetraphenyporphyrin cation radical perchlorate with nucleophiles. J Org Chem 44:4069–4075
Smith AB (1972) Colorimetric indicator for the detection of nitrogen dioxide. US 3,681,027
Tanaka T, Ohyama T, Maruo YY, Hayashi T (1998) Coloration reactions between NO2 and organic compounds in porous glass for cumulative gas sensor. Sens Actuators B 47:65–69
Wang C, Yin L, Zhang L, Xiang D, Gao R (2010) Metal oxide gas sensors: sensitivity and influence factors. Sensors 10:2088–2106
Wayland BB, Olson LW (1974) Spectroscopic studies and bonding model for nitric oxide complexes of iron porphyrins. J Am Chem Soc 96(19):6037–6041
Yamazoe N, Shimanoe K (2009) New perspectives of gas sensor technology. Sens Actuators B 138:100–107
Acknowledgments
This research has been supported by the internal programs of the Fraunhofer Gesellschaft (WISA Food Chain Management) and the Spitzencluster Microtec Südwest in cooperation with the Federal Ministry of Education and Research (BMBF) (project SensRFID).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peter, C., Schmitt, K., Apitz, M. et al. Metallo-porphyrin zinc as gas sensitive material for colorimetric gas sensors on planar optical waveguides. Microsyst Technol 18, 925–930 (2012). https://doi.org/10.1007/s00542-011-1412-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-011-1412-x