Abstract
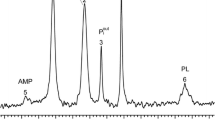
31P and23Na nuclear magnetic resonance (NMR) spectroscopy was employed to study the dynamic changes in intracellular high-energy phosphates and sodium during 15 min of forebrain ischemia and recirculation inin vivo rat brain. In the presence of the shift reagent Dysprosium triethylenetetramine-N,N,N′, N″, N‴,N‴-hexaacetic and [Dy(TTHA)], the sodium peak separated into two peaks, unshifted and shifted. During 15 min of ischemia, the unshifted sodium peak decreased and the shifted sodium peak increased. With recirculation, the unshifted and the shifted sodium peaks returned to the preischemia level within 10 min, but the shifted one increased during 30–60 min. Intracellular high-energy phosphates and intracellular pH (pHi) decreased during 15 min of ischemia and returned to the preischemia levels within 20 min of recirculation. We conclude that the decrease in unshifted sodium peak during ischemia is due to the decrease in subarachnoid sodium and the cellular influx of interstitial sodium would be minimum. The increase in shifted sodium peak during ischemia is considered to be due to the dilatation of cerebral blood vessels and the increase in interstitial sodium which was transported from subarachnoid space.
Similar content being viewed by others
References
Kurata M, Okuda M, Muneyuki M, et al: [31P]MRS study of the protective effects of prostaglandin oligomers on forebrain ischemia in rats. Brain Res 545:315–318, 1991
Okuda M, Muneyuki M, Kawarada Y, et al: Effects of portal vein occlusion on high-energy phosphates in rat liver measured by31P-NMR spectroscopy. Dig Surg 6:176–179, 1989
Naritomi H, Kanashiro M, Sasaki M, et al:In vivo measurements of intra- and extracellular Na+ and water in the brain and muscle by nuclear magnetic resonance spectroscopy with shift reagent. Biophys J 52:611–616, 1987
Blum H, Schnall MD, Chance B, et al: Intracellular sodium flux and highenergy phosphorus metabolites in ischemic skeletal muscle. Am J Physiol 255:C377-C384, 1988
Blum H, Osbakken MD, Johnson RG Jr: Sodium flux and bioenergetics in the ischemic rat liver. Magn Reson Med 18:348–357, 1991
Eliff SM, Maruki Y, Monsein LH, et al: Sodium, ATP and intracellular pH transient during reversible complete ischemia of dog cerebrum. Stroke 22:233–241, 1991
Pike MM, Frazer JC, Dedrick DF, et al:23Na and39K nuclear magnetic resonance studies of perfused rat hearts. Discrimination of intraand extracellular ions using a shift reagent. Biophys J 48:159–173, 1985
Gupta RK, Gupta P: Direct observation of resolved resonances from intra- and extracellular sodium-23 ions in NMR studies of intact cells and tissues using dysprosium (III) tripolyphosphate as paramagnetic shift reagent. J Magn Reson 47:344–350, 1982
Naritomi H: Validity ofin vivo nuclear magnetic resonance methods in measurement of intracellular water and sodium. Biophys J 54:193, 1988
Sutherland GR, Lesiuk H, Bose R, et al: Effect of mannitol, nimodipine, and indomethacin singly or in combination on cerebral ischemia in rats. Stroke 19:571–578, 1987
Petroff OAC, Prichard JW, Behar KL, et al: Cerebral intracellular pH by31P nuclear magnetic resonance spectroscopy. Neurology 35:781–788, 1985
Winer BJ: Statistical Principles in Experimental design. New York, McGraw-Hill, 1971
Hansen AJ: Effect of anoxia on ion distribution in the brain. Physiol Rev 65:101–148, 1985
Cremer JE, Seville MP: Regional brain blood flow, blood volume, and hematocrit values in the adult rat. J Cereb Blood Flow Metab 3:254–256, 1983
Springer CS Jr: Measurement of metal cation compartmentalization in tissue by high-resolution metal cation NMR. Annu Rev Biophys Chem 16:375–399, 1987
Hossman K-A: Development and resolution of ischemic brain swelling. In Pappius HM, Feindel W (eds) Dynamics of brain edema, SpringerVerlag, Berlin, 1976, pp. 219–227
Hossmann K-A, Sakaki S, Zimmermann V: Cation activities in reversible ischemia of the cat brain. Stroke 8:77–81, 1977
Tomita M, Gotoh F, Tanahashi N, et al: Pressure-flow relationship of the brain below autoregulatory range. Neurol India 37(suppl):26, 1989
Mellergard P, Bengtsson F, Smith ML, et al: Time course of early brain edema following reversible forebrain ischemia in rats. Stroke 20:1565–1570, 1989
Author information
Authors and Affiliations
About this article
Cite this article
Kurata, M. 31P and23Na nuclear magnetic resonance study on forebrain ischemia in rats with shift reagent Dy(TTHA). J Anesth 7, 325–333 (1993). https://doi.org/10.1007/s0054030070325
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s0054030070325