Abstract
Purpose
This systematic review and meta-analysis aimed to evaluate the association between intraoperative oliguria and the risk of postoperative acute kidney injury (AKI) in patients undergoing non-cardiac surgery.
Methods
The MEDLINE and EMBASE databases were searched up to August 2022 for studies in adult patients undergoing non-cardiac surgery, where the association between intraoperative urine output and the risk of postoperative AKI was assessed. Both randomised and non-randomised studies were eligible for inclusion. Study selection and risk of bias assessment were independently performed by two investigators. The risk of bias was evaluated using the Newcastle–Ottawa scale. We performed meta-analysis of the reported multivariate adjusted odds ratios for the association between intraoperative oliguria (defined as urine output < 0.5 mL/kg/hr) and the risk of postoperative AKI using the inverse-variance method with random effects models. We conducted sensitivity analyses using varying definitions of oliguria as well as by pooling unadjusted odds ratios to establish the robustness of the primary meta-analysis. We also conducted subgroup analyses according to surgery type and definition of AKI to explore potential sources of clinical or methodological heterogeneity.
Results
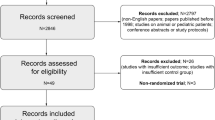
Eleven studies (total 49,252 patients from 11 observational studies including a post hoc analysis of a randomised controlled trial) met the selection criteria. Seven of these studies contributed data from a total 17,148 patients to the primary meta-analysis. Intraoperative oliguria was associated with a significantly elevated risk of postoperative AKI (pooled adjusted odds ratio [OR] 1.74; 95% confidence interval [CI] 1.36–2.23, p < 0.0001, 8 studies). Sensitivity analyses supported the robustness of the primary meta-analysis. There was no evidence of any significant subgroup differences according to surgery type or definition of AKI.
Conclusions
This study demonstrated a significant association between intraoperative oliguria and the risk of postoperative AKI, regardless of the definitions of oliguria or AKI used. Further prospective and multi-centre studies using standardised definitions of intraoperative oliguria are required to define the thresholds of oliguria and establish strategies to minimise the risk of AKI.




Similar content being viewed by others
Data availability
All datasets utilised in the analysis during the current study are available from the corresponding author on reasonable request.
References
Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–8.
Eknoyan G. The origins of nephrology–galen, the founding father of experimental renal physiology. Am J Nephrol. 1989;9:66–82.
Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–61.
Bellomo R, Roneo C, Kellum J, Mehta RL, Palevsky P. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8:R204–12.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Warnock DG, Levin A. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
Kidney Disease: Improving Global Outcomes (KIDGO). Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int. 2012; Suppl 2;1–138.
Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26:509–15.
Gameiro J, Neves JB, Rodrigues N, Bekerman C, Melo MJ, Pereira M, Teixeira C, Mendes I, Jorge S, Rosa R, Lopes JA. Acute kidney injury, long-term renal function and mortality in patients undergoing major abdominal surgery: a cohort analysis. Clin Kidney J. 2016;9:192–200.
Kullmar M, Meersch M. intraoperative oliguria: physiological or beginning acute kidney injury? Anesth Analg. 2018;127:1109–10.
du Toit L, Biccard BM. The relationship between intraoperative oliguria and acute kidney injury. Br J Anaesth. 2019;122:707–10.
Fukazawa K, Lee HT. Volatile anesthetics and AKI: risks, mechanisms, and a potential therapeutic window. J Am Soc Nephrol. 2014;25:884–92.
Nguyen NT, Perez RV, Fleming N, Rivers R, Wolfe BM. Effect of prolonged pneumoperitoneum on intraoperative urine output during laparoscopic gastric bypass. J Am Coll Surg. 2002;195:476–83.
Kork F, Balzer F, Spies CD, Wernecke KD, Ginde AA, Janowski J, Eltzchig HK. Minor postoperative increases of creatinine are associated with higher mortality and longer hospital stay in surgical patients. Anesthesiology. 2015;123:1301–11.
McIlroy DR, Belloma R, Billings FT, Karkouti K, Prowle JR, Shaw AD, Myles PS. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine (StEP) initiative: renal endpoints. Br J Anaesth. 2018;121:1013–24.
O’Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20:187.
Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, Szabo Z, Kalantar-Zadeh K, Kovesdy CP. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis. 2016;67:872–80.
Thakar CV, Kharat V, Blank S, Leonard AC. Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol. 2007;2:426–30.
Cabezuelo JB, Ramirez P, Rios A, Acosta F, Torres D, Sansano T, Pons JA, Bru M, Montoya M, Bueno FS, Robles R. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006;69:1073–80.
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–8.
Hultstrom M. Neurohormonal interactions on the renal oxygen delivery and consumption in haemorrhagic shock-induced acute kidney injury. Acta Physiol (Oxf). 2013;209:11–25.
Fani F, Regolisti G, Delsante M, Cantalappi V, Castellano G, Gesualdo L, Villa G, Fiaccadori E. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J Nephrol. 2018;31:351–9.
Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14:217–30.
Goren O, Matot I. (2015) Perioperative acute kidney injury. Br J Anaesth. 115 Suppl 2: ii3–14.
Burton D, Nicholson G, Hall G. Endocrine and metabolic response to surgery. Continuing Education Anaesthesia Critical Care Pain. 2004;4:169–71.
Philbin DM, Coggins CH. Plasma antidiuretic hormone levels in cardiac surgical patients during morphine and halothane anesthesia. Anesthesiology. 1978;49:95–8.
Bozkurt P, Kaya G, Yuksel Y, Fatius A, Bakan M, Hacibekiroglu M, Kavunoblu G. Effects of systemic and epidural morphine on antidiuretic hormone levels in children. Paediatr Anaesth. 2003;13:508–14.
Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805.
Nash DM, Mustafa RA, McArthur E, Wijeysundera DN, Paterson JM, Sharan S, Vinden C, Wald R, Welk B, Sessler DI, Devereaux PJ. Combined general and neuraxial anesthesia versus general anesthesia: a population-based cohort study. Can J Anaesth. 2015;62:356–68.
Klein SJ, Lehner GF, Forni LG, Joannidis M. Oliguria in critically ill patients: a narrative review. J Nephrol. 2018;31:855–62.
Chiu AW, Chang LS, Birkett DH, Babayan RK. Changes in urinary output and electrolytes during gaseous and gasless laparoscopy. Urol Res. 1996;24:361–6.
Lucas GNC, Leitao AC, Alencar RL, Xavier RM, Daher ED, Junior GB. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. J Bras Nefrol. 2019;41:124–30.
Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45.
Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–12.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff J, Mulrow C, Moher D. The PRISMA statement for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–12.
Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). (2022) Cochrane Handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Myles PS, McIIroy DR, Bellomo R, Wallace S. Importance of intraoperative oliguria during major abdominal surgery: findings of the restrictive versus liberal fluid therapy in major abdominal surgery trial. Br J Anaesth. 2019;122:726–33.
Kheterpal S, Tremper KK, Englesbe MJ, O’Reilly M, Shanks AM, Fetterman DM, Rosenberg AL, Swartz RD. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892–902.
Mizota T, Yamamoto Y, Hamada M, Matsukawa S, Shimizu S, Kai S. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br J Anaesth. 2017;119:1127–34.
Zhao BC, Lei SH, Yang X, Zhang Y, Qiu SD, Liu WF, Li C, Liu KX. Assessment of prognostic value of intraoperative oliguria for postoperative acute kidney injury: a retrospective cohort study. Br J Anaesth. 2020;126:799–807.
Inacio R, Gameiro J, Amaro S, Duarte M. Intraoperative oliguria does not predict postoperative acute kidney injury in major abdominal surgery: a cohort analysis. J Bras Nefrol. 2021;43:9–19.
Goren O, Levy A, Cattan A, Lahat G, Matot I. Acute kidney injury in pancreatic surgery; association with urine output and intraoperative fluid administration. Am J Surg. 2017;214:246–50.
Valencia Morales DJ, Plack DL, Kendrick ML, Schroeder DR, Sprung, J, Weingarten TN. (2022) Urine output and acute kidney injury following laparoscopic pancreas operations. HPB, Article in press
Kim WH, Lee HC, Lim L, Ryu HG, Jung CW. Intraoperative oliguria with decreased SvO2 predicts acute kidney injury after living donor liver transplantation. J Clin Med. 2018;8(29):1–14.
Slankamenac K, Beck-Schimmer B, Breitenstein S, Puhan MA, Clavien PA. Novel prediction score including pre- and intraoperative parameters best predicts acute kidney injury after liver surgery. World J Surg. 2013;37(11):2618–28.
Hur M, Park SK, Yoo S, Choi SN, Jeong CW, Kim WH, Kim JT, Kwak C, Bahk JH. The association between intraoperative urine output and postoperative acute kidney injury differs between partial and radical nephrectomy. Sci Rep. 2019;9(760):1–9.
Shiba A, Uchino S, FujiiT Takinami M, Uezono S. Association between intraoperative oliguria and acute kidney injury after major noncardiac surgery. Anesth Analg. 2018;127:1229–35.
Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829–35.
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J. Carpenter j, Rucker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peter J, Macaskill P, Schwarzer G, Duval s, Altman DG, Moher d, H'iggins JPT. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ.;343 4002
Puckett JR, Pickering JW, Palmer SC, McCall JL, Kluger MT, De Zoysa J, Endre ZH, Soop M. Low versus standard urine output targets in patients undergoing major abdominal surgery: a randomized noninferiority trial. Ann Surg. 2017;265(5):874–81.
Prowle JR, Liu YL, Licari E, Bagshaw SM,Egi M, Haase M, Haase-Fielitz A, Kellum JA, Cruz D, Ronco C, Tsutsui K, Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care. 2011;15:R172. https://doi.org/10.1186/cc10318.
Md Ralib A, Pickering JW, Shaw GM, Endre ZH. The urine output definition of acute kidney injury is too liberal. Crit Care. 2013;17:R112.
Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W. A European Renal Best Practice (ERBP) position statement on Kidney Disease Improving Global Outcome (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast induced nephropathty. Nephrol Dial Transpl. 2012;27:4263–72.
Funding
None.
Author information
Authors and Affiliations
Contributions
DAM contributed to study conception, acquisition, analysis, interpretation of data, drafting, and editing of the manuscript. SSL contributed to analysis, interpretation of data, drafting and editing of the manuscript. SGKO contributed to acquisition, analysis, interpretation of data, drafting, and editing of the manuscript. PCAK provided oversight and expertise on all aspects of the manuscript including study conception, acquisition, analysis, interpretation of data, drafting, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
540_2022_3150_MOESM1_ESM.pptx
Supplementary file1 Sensitivity Analysis: Odds ratio of Acute Kidney Injury according to the presence of oliguria as defined by the study (PPTX 99 KB)
540_2022_3150_MOESM2_ESM.pptx
Supplementary file2 Sensitivity Analysis: Odds ratio of Acute Kidney Injury according to the presence of oliguria (<0.3 mL/kg/hr) (PPTX 90 KB)
540_2022_3150_MOESM3_ESM.pptx
Supplementary file3 Sensitivity Analysis: Odds ratio of Acute Kidney Injury according to the presence of oliguria (<0.5 mL/kg/hr) using pooled unadjusted data (PPTX 84 KB)
540_2022_3150_MOESM4_ESM.pptx
Supplementary file4 Subgroup Analysis according to surgery type, to identify any subgroup differences between patients undergoing nephrectomy (partial or total) and all other types of surgery: Odds ratio of Acute Kidney Injury according to according to the presence of oliguria (<0.5 mL/kg/hr) (PPTX 114 KB)
540_2022_3150_MOESM5_ESM.pptx
Supplementary file5 Subgroup Analysis according to surgery type: Odds ratio of Acute Kidney Injury according to the presence of oliguria (<0.5mL/kg/hr) (PPTX 128 KB)
540_2022_3150_MOESM6_ESM.pptx
Supplementary file6 Subgroup Analysis according to definition of Acute Kidney Injury: Odds ratio of AKI according to according to the presence of oliguria (<0.5 mL/kg/hr) (PPTX 123 KB)
About this article
Cite this article
Milder, D.A., Liang, S.S., Ong, S.G.K. et al. Association between intraoperative oliguria and postoperative acute kidney injury in non-cardiac surgical patients: a systematic review and meta-analysis. J Anesth 37, 219–233 (2023). https://doi.org/10.1007/s00540-022-03150-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-022-03150-8