Abstract
Purpose
To assess the effects of various concentrations of dexmedetomidine on the human blood coagulation profile using rotational thromboelastometry (ROTEM).
Methods
Venous blood samples were collected from 11 healthy volunteers and divided into four specimen bottles; dexmedetomidine was added to attain final sample concentrations of 0, 0.5, 1.0, and 1.5 ng/mL. ROTEM was performed on each study sample.
Results
The concentration of dexmedetomidine increased, and the ROTEM values showed a hypercoagulable state. The change in clotting time (CT) for INTEM was larger in samples with a dexmedetomidine concentration of 1.5 ng/mL (− 34%) than in the 0.5 ng/mL samples (− 16%) (P = 0.010). The change in clot formation time (CFT) for INTEM was greater in 1.5 ng/mL samples (− 16%) than in 0.5 ng/mL samples (− 4%) (P = 0.004). A greater decrease in CT for EXTEM was identified in the 1.0 ng/mL and 1.5 ng/mL samples (− 36% and − 37%, respectively) than in the 0.5 ng/mL samples (− 12%) (P = 0.003 for both categories). The change in CFT for EXTEM was greater in the 1.0 ng/mL and 1.5 ng/mL samples (− 11% and − 13%, respectively) than in the 0.5 ng/mL samples (− 4%) (P = 0.006 and P = 0.001, respectively). A bigger change in maximum clot firmness (MCF) for EXTEM was observed in the 1.5 ng/mL samples (4%) than in the 0.5 ng/mL samples (0%) (P = 0.002). The change in MCF for FIBTEM was greater in the 1.5 ng/mL samples (19%) than in the 0.5 ng/mL samples (5%) (P = 0.001).
Conclusions
All coagulation pathways showed a hypercoagulable state as the concentration of dexmedetomidine increased. Nevertheless, most of the values of ROTEM were maintained within the reference ranges.
Clinical Trial NCT04269278.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dexmedetomidine, a highly selective α2-specific agonist, has an α2 receptor affinity of 1620:1. Owing to this characteristic action, dexmedetomidine has analgesic and hypnotic properties, elicited via α2-receptor stimulation in the dorsal horn of the spinal cord and locus coeruleus, respectively [1,2,3], which has rendered it a possibility for procedural sedation or intensive-care unit sedation [4, 5].
Studies have shown that human platelets have approximately 250–300 α2-adrenoceptors on their surface, and thus, it has been postulated that the stimulation of α2-adrenoceptors by dexmedetomidine or epinephrine, in vitro, may induce platelet aggregation [6, 7]. However in in vivo studies, dexmedetomidine increases nitric oxide (NO) release in the vascular endothelium [3] and attenuates catecholamine release through negative feedback by pre-synaptic α2-adrenoceptor stimulation [4], thereby reducing platelet aggregation [3]. The hypnotics drug dexmedetomidine, which is used for sedation and anxiolysis during surgery, can also hinder the coagulation cascade by attenuating the sympathetic tone [8]. Clinically, dexmedetomidine caused an attenuation of the activation of blood coagulation after surgery [9]. On the other hand, decreased the amount of intraoperative bleeding was also reported in functional endoscopic sinus surgery [10]. To sum up these studies, the final effects of dexmedetomidine resulting from the interaction of these factors with blood coagulation are still unknown. Due to the possibility of hemostatic changes that can result from drug interactions, it is important to determine the effects of dexmedetomidine on platelet function and blood coagulation because of the risk of additive effects with other medications on the coagulation pathway.
The purpose of this study was to assess the effects of different concentrations of dexmedetomidine on hemostatic properties using rotational thromboelastometry (ROTEM), which provides information of coagulation factor activity and platelet function [11].
Methods
Ethics approval was obtained from the Institutional Review Board of Seoul National University Bundang Hospital, Seongnam-si, South Korea (approval granted on February 11, 2020; B-2002/595-301), and was registered in the clinical trial database (https://clinicaltrials.gov/, NCT04269278). This study was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from five male and six female volunteers (age range, 28–40 years), who were healthy and did not take any agents known to inhibit hemostasis, such as antiplatelet, anticoagulant, and nonsteroidal anti-inflammatory drugs.
Ten milliliters of venous blood were individually drawn from an antecubital vein using 18-gauge needle and immediately placed into a citrate-containing polypropylene tube (Vacutainer®, Becton Dickinson, Plymouth, UK). A two-syringe technique was used for blood sampling to decrease the risk of tissue thromboplastin contamination: after discarding the first blood sample, the blood sample to be used in this study was taken immediately. Furthermore, the collected samples were split into four bottles, each containing 2.0 mL of blood. According to the findings from a previous study, when dexmedetomidine is administered at a rate of 0.5 μg/kg/h, the predicted steady-state concentration is around 0.49–1.15 ng/mL according to different patient characteristics such as age, plasma albumin, and total body weight [12]. Consequently, we prepared blood samples containing dexmedetomidine at three different concentrations (0.5, 1.0, and 1.5 ng/mL). To generate the final sample concentrations, 0.25, 0.5, and 0.75 μL of dexmedetomidine (Hospira INC, North Carolina, USA) were added to three bottles, respectively, after the same volume of blood was discarded. In the fourth bottle, no dexmedetomidine was added to the blood sample, and this sample was used as the baseline value (control).
The ROTEM analysis was conducted by one investigator, and the ROTEM parameters, such as CT, CFT, and MCF, were determined. Using the recommended reagents, we obtained the FIBTEM, EXTEM, and INTEM values, which reflect the status of the fibrin polymerization and the extrinsic and intrinsic processes of the coagulation pathways, respectively.
Based on previous studies [13,14,15], assuming a 20% difference in the CFT of EXTEM from the baseline values and aiming for a power of 80% at the 5% significance level, 11 participants were estimated, anticipating a dropout rate of 15%.
The obtained data were expressed as the mean (standard deviation) and as percentages. Data were analyzed using SPSS for Windows (ver. 22; IBM Corp., Armonk, NY, USA), and a P value of < 0.05 was taken to indicate statistical significance. Repeated-measured analysis of variance was used to determine whether there was a change in mean ROTEM values according to the dexmedetomidine concentration. Thereafter, differences in each ROTEM value among the concentrations were analyzed using a paired t test. For this multiple comparison adjusted by Bonferroni correction, a P value < 0.017 (0.05/3) was considered statistically significant.
Results
The ROTEM values showed a hypercoagulable tendency as the concentration of dexmedetomidine increased (Table 1). Significant differences were observed among the concentrations (P < 0.01). Based on the post hoc comparisons, there were significant changes in all CT, CFT, and MCF values of INTEM in the 1.0 ng/mL and 1.5 ng/mL samples compared to those in the control. The CT for INTEM decreased significantly with 0.5 ng/mL dexmedetomidine. Regarding EXTEM, CT and CFT decreased significantly at 0.5 ng/mL dexmedetomidine; however, MCF only showed a significant change at 1.5 ng/mL. In addition, the MCF of FIBTEM increased significantly in the 1.0 and 1.5 ng/mL samples. However, all mean values of the ROTEM parameters were within the normal reference ranges (Table 1).
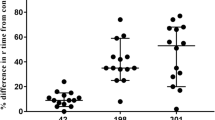
Figure 1A depicts the percentage change in the INTEM parameters from the baseline value in each concentration category. Significant differences were observed among the concentrations (P < 0.01). Based on the post hoc comparisons, the change in the CT for INTEM was larger in blood samples containing 1.5 ng/mL of dexmedetomidine (− 34%) than in the 0.5 ng/mL samples (− 16%) (P = 0.010). The change in the CFT for INTEM was greater in the 1.5 ng/mL samples (− 16%) than in the 0.5 ng/mL samples (− 4%) (P = 0.004). There were no significant differences among the concentration categories with respect to the change in the MCF for INTEM (P = 0.294).
Percentage change from baseline values for the following rotational thromboelastometry (ROTEM) parameters: A INTEM, B EXTEM, and C FIBTEM for the 0.5 ng/mL (black), 1.0 ng/mL (gray), and 1.5 ng/mL (white) samples. Values are the means (standard deviations). CT clotting time (s), CFT clot formation time (s), MCF maximum clot firmness (mm). *Significantly different from the 0.5 ng/mL sample (P < 0.017)
Figure 1B depicts the % change in the EXTEM parameters from the baseline value in each concentration category. A significant decrease in the CT for EXTEM was identified in the 1.0 and 1.5 ng/mL dexmedetomidine samples (− 36% and − 37%, respectively) compared with the 0.5 ng/mL samples (− 12%) (P = 0.003 in both). The change in the CFT for EXTEM was also significant in the 1.0 and 1.5 ng/mL samples (− 11% and − 13%, respectively) compared to the 0.5 ng/mL samples (− 4%) (P = 0.006 and P = 0.001, respectively). A significant change in the MCF for EXTEM was identified in the 1.5 ng/mL samples (4%) compared to that observed in the 0.5 ng/mL samples (0%) (P = 0.002).
The change in the MCF for FIBTEM was greater for the samples containing 1.5 ng/mL of dexmedetomidine (19%) than for the samples containing 0.5 ng/mL (5%) (P = 0.001, Fig. 1C).
Discussion
In this study, we investigated blood coagulation levels through ROTEM analyses using different concentrations of dexmedetomidine. The results showed that the coagulation pattern changed toward a hypercoagulable state when the concentration of dexmedetomidine was increased. All mean results obtained from the ROTEM tests, however, were within the normal reference ranges, even though there was a tendency to change to a hypercoagulable state.
Several clinical trials have assessed the relationship between dexmedetomidine and blood coagulation [9, 16] or intraoperative bleeding [10, 17]. Gousheh et al. [10] reported that dexmedetomidine administration reduced the amount of bleeding during functional endoscopic sinus surgery. Chen et al. [9] analyzed the blood coagulation system using thromboelastography (TEG) after open radical gastrectomy when dexmedetomidine was used as an adjuvant drug during total intravenous anesthesia. They found that administration of dexmedetomidine caused an attenuation of the activation of blood coagulation following surgery. Martins et al. [16] evaluated the changes in blood coagulation using TEG in surgical patients. In their study, they compared the change in TEG parameters among three groups, infused with saline (control), dexmedetomidine and midazolam, and reported mild hypocoagulation by dexmedetomidine. In an in vitro study, Kawamoto et al. [7] performed platelet function analysis using aggregometers to assess the change in platelet function after exposure to dexmedetomidine and showed both suppressive and enhancing effects through their action on the I1-imidazoline and α2-adrenergic receptors, respectively.
These conflicting results, including the new findings illustrated in this study, may have been due to diversity in the methods used in each study, such as in vivo vs. in vitro, healthy volunteers vs. patients, surgical vs. nonsurgical conditions and rotational thromboelastometry vs. thromboelastography. Different clinical conditions, such as intraoperative bleeding, changes in coagulation factors during surgery [18], stress response to surgery [19], type of anesthetic (propofol, volatile agents) used, and fluid administration may influence the blood coagulation system.
Although dexmedetomidine is expected to affect platelet function as a selective α2-adrenoceptor agonist, the exact mechanism of action remains unclear. One suggested function by Kawamoto et al. [7] was that dexmedetomidine enhances human platelet aggregation via the activation of α2-adrenoceptors on platelets. Platelet activation is known to promote blood coagulation interactively and dependently [20].
Interestingly, all coagulation pathways analyzed by ROTEM were influenced by dexmedetomidine. Each ROTEM parameter that was tested represents a different coagulation step. CT indicates the rate of thrombin generation and is primarily influenced by intrinsic or extrinsic coagulation factors [21] which mainly depends on the generation of thrombin, platelet count and function [21], and is dependent on the initial speed of fibrin polymerization. MCF refers to the clot stability by polymerized fibrin and thrombocytes [11].
By providing binding sites for prothrombin and factor XI, platelets can support the initiation phase of coagulation [20]. In the intrinsic coagulation pathway, factors XII, XI, and IX are involved, and factor IX is activated by the platelet-bound factor XIa [22]. The changes in INTEM parameters, including a shortened CT, CFT, and an increased MCF, may reflect the involvement of platelets activated by dexmedetomidine on the initiation and propagation of the coagulation cascade. In addition, activated platelets are known to have an initiating role in tissue factor and factor VIIa in coagulation activation, which is involved in the extrinsic coagulation pathway [23]. Furthermore, the MCF for EXTEM indicates the tensile strength of the whole-blood clot, which reflects the interaction between polymerized fibrin and thrombin-activated platelets through GP IIb/IIIa receptors on the surface of platelets [24]. Accordingly, the clot strength in EXTEM depends on platelet function and count [25]. In the present study, CT, CFT, and MCF values following INTEM and EXTEM analyses showed significant changes and the effect of dexmedetomidine was prominent at higher drug concentrations.
Whereas the ROTEM MCF value reflects the overall platelet function and fibrinogen levels [26], FIBTEM MCF evaluates the contribution of functional fibrinogen to clot formation after inhibiting platelets. Obviously, fibrin clot formation is insufficient without platelets, so FIBTEM MCF trace reaches only within an amplitude of 25 mm. Interestingly, a gradual increase of MCF was observed in FIBTEM by increasing the concentration of dexmedetomidine compared with the baseline value. Little is known about the effect of dexmedetomidine on the fibrinogen. Further study is needed to identify the role of α2-adrenergic agonists in the thrombin generation step, where the fibrinogen participated, without platelet.
The present study has several limitations. First, the findings may not be relevant to clinical fields because of the characteristics of in vitro studies. However, the positive aspects of in vitro experiments are reproducibility and controllability. These properties of an experimental study could provide credibility to the results of this study. Second, since this in vitro study was intended to assess the effects of single drug on blood coagulation, the effects of additional consumptive coagulopathy and associated hyperfibrinolysis associated with exposure to damaged tissue components caused by surgical procedures or severe trauma cannot be determined. It is also too early to discuss whether dexmedetomidine is prohibited or not in patients with risks of thrombosis. Further study is required to understand how dexmedetomidine can change the coagulation profile in clinical hypercoagulable or hypocoagulable states shown in various critically ill and septic condition.
In conclusion, as evaluated by ROTEM, this study showed that dexmedetomidine may affect blood coagulation cascades (change toward a hypercoagulable state). However, the changes in blood coagulation were within the acceptable reference range, indicating that sedation using dexmedetomidine may not be harmful in terms of coagulopathy when administered within the therapeutic range.
References
Hayashi Y, Maze M. Alpha 2 adrenoceptor agonists and anaesthesia. Br J Anaesth. 1993;71:108–18.
Aantaa R, Scheinin M. Alpha 2-adrenergic agents in anaesthesia. Acta Anaesthesiol Scand. 1993;37:433–48.
Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–65.
Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent). 2001;14:13–21.
Naaz S, Ozair E. Dexmedetomidine in current anaesthesia practice—a review. J Clin Diagn Res. 2014;8:GE01–4.
Lanza F, Beretz A, Stierle A, Hanau D, Kubina M, Cazenave JP. Epinephrine potentiates human platelet activation but is not an aggregating agent. Am J Physiol. 1988;255:H1276–88.
Kawamoto S, Hirakata H, Sugita N, Fukuda K. Bidirectional effects of dexmedetomidine on human platelet functions in vitro. Eur J Pharmacol. 2015;766:122–8.
Misiolek H, Wojcieszek E, Dyaczynska-Herman A. Comparison of influence of thiopentone, propofol and midazolam on blood serum concentration of noradrenaline and cortisol in patients undergoing non-toxic struma operation. Med Sci Monit. 2000;6:319–24.
Chen Z, Shao DH, Mao ZM, Shi LL, Ma XD, Zhang DP. Effect of dexmedetomidine on blood coagulation in patients undergoing radical gastrectomy under general anesthesia: a prospective, randomized controlled clinical trial. Medicine (Baltimore). 2018;97: e11444.
Gousheh SMR, Olapour AR, Nesioonpour S, Rashidi M, Pooyan S. The effect of intravenous infusion of dexmedetomidine to prevent bleeding during functional endoscopic sinus surgery: a clinical trial. Anesth Pain Med. 2017;7: e12682.
Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89:228–32.
Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56:893–913.
Shin HJ, Lee H, Na HS. The effect of a mixture of 2.7% sorbitol-0.54% mannitol solution on blood coagulation: an invitro, observational healthy volunteer study using rotational thromboelastometry (ROTEM). Korean J Anesthesiol. 2019;72:143–9.
Shin HJ, Park HY, Na HS, Hong JP, Lee GW, Do SH. The effects of Plasma-Lyte 148 solution on blood coagulation: an in-vitro, volunteer study using rotational thromboelastometry. Blood Coagul Fibrinolysis. 2018;29:446–50.
Shin HJ, Na HS, Lee S, Lee GW, Do SH. The effect of hyperglycemia on blood coagulation: in vitro, observational healthy-volunteer study using rotational thromboelastometry (ROTEM). Medicine (Baltimore). 2016;95: e4703.
Martins CR, Tardelli MA, Amaral JL. Effects of dexmedetomidine on blood coagulation evaluated by thromboelastography. Rev Bras Anestesiol. 2003;53:705–19.
Mizrak A, Karatas E, Saruhan R, Kara F, Oner U, Saricicek V, Baysal E. Does dexmedetomidine affect intraoperative blood loss and clotting tests in pediatric adenotonsillectomy patients? J Surg Res. 2013;179:94–8.
Lison S, Weiss G, Spannagl M, Heindl B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis. 2011;22:190–6.
Naito Y, Tamai S, Shingu K, Shindo K, Matsui T, Segawa H, Nakai Y, Mori K. Responses of plasma adrenocorticotropic hormone, cortisol, and cytokines during and after upper abdominal surgery. Anesthesiology. 1992;77:426–31.
Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186–93.
Görlinger K, Dirkmann D, Hanke AA. Rotational Thromboelastometry (ROTEM®). Berlin: Springer; 2016. p. 267–98.
Gailani D, Renne T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–12.
Lau HK. The interaction between platelets and factor VII/VIIa. Transfus Apher Sci. 2003;28:279–83.
Tanaka KA, Bolliger D, Vadlamudi R, Nimmo A. Rotational thromboelastometry (ROTEM)-based coagulation management in cardiac surgery and major trauma. J Cardiothorac Vasc Anesth. 2012;26:1083–93.
Gonzalez E, Moore EE, Moore HB, Chapman MP, Silliman CC, Banerjee A. Trauma-induced coagulopathy: an institution’s 35 year perspective on practice and research. Scand J Surg. 2014;103:89–103.
Korpallova B, Samos M, Bolek T, Skornova I, Kovar F, Kubisz P, Stasko J, Mokan M. Role of thromboelastography and rotational thromboelastometry in the management of cardiovascular diseases. Clin Appl Thromb Hemost. 2018;24:1199–207.
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Shin, HJ., Boo, G. & Na, HS. Effects of dexmedetomidine on blood coagulation: an in vitro study using rotational thromboelastometry. J Anesth 35, 633–637 (2021). https://doi.org/10.1007/s00540-021-02969-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-021-02969-x