Abstract
Purpose
Previous studies evidenced that orotracheal intubation without neuromuscular blockers is feasible in children and has some potential advantages. Remifentanil has favorable pharmacodynamic and pharmacokinetic properties as an opioid for orotracheal intubation, but its dose for excellent intubation conditions when co-administered with propofol has not been established. This study was designed to find the minimum effective dose of remifentanil for excellent intubation conditions of children when co-administered with propofol, without neuromuscular relaxant drugs.
Method
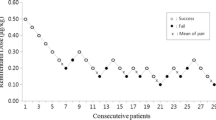
Blinded adaptive clinical trial, with sequential allocation of 27 children between 2 and 9 years-old, American Society of Anesthesiologists’ physical status PI or PII, scheduled for elective surgery under general anesthesia. Remifentanil dose began at 2 µg/kg and varied by 0.25 µg/kg according to the sequential allocation up-and-down rule designed by Dixon and Massey. Remifentanil was infused in 30 s and followed by propofol (3 mg/kg) in 20 s. Laryngoscopy and intubation were performed and assessed using Viby-Mogensen criteria, 90 s after the end of opioid administration. Inclusion of patients stopped after six crossovers, and remifentanil effective dose was estimated using pooled adjacent-violators algorithm.
Results
Remifentanil effective dose for 50% was established in 3.04 µg/kg (IC 95% 2.68–3.11, p < 0.05). The most frequent adverse effect was difficult positive pressure facial mask ventilation, which occurred in four children (15%).
Conclusion
Minimum remifentanil effective dose for providing excellent intubating conditions when co-administered with a single standard dose of propofol without the use of neuromuscular blockers in children is 3.04 µg/kg.
Trial registration
NCT02454868.


Similar content being viewed by others
References
Bouvet L, Stoian A, Rimmelé T, Allaouchiche B, Chassard D, Boselli E. Optimal remifentanil dosage for providing excellent intubating conditions when co-administered with a single standard dose of propofol. Anaesthesia. 2009;64:719–26.
Erhan E, Ugur G, Gunusen I, Alper I, Ozyar B. Propofol—not thiopental or etomidate—with remifentanil provides adequate intubating conditions in the absence of neuromuscular blockade. Can J Anesthesia. 2003;50:108–15.
Fiacchino F. Rhabdomyolysis succinylcholine. Anesthesiology. 1996;84:480.
Todd MM. Succinylcholine hyperkalemia after burns. Anesthesiology. 1999;91:321.
Boba A. Succinylcholine in children. Anesthesia Analg. 1988;67:800.
Kalow W. Succinylcholine and malignant hyperthermia. Surv Anesthesiol. 1973;17:358–9.
Lee YJ. Succinylcholine and muscie pain. Korean J Anesthesiol. 1980;13:391.
Batra YK, Qattan A, Ali AR, Qureshi SS, Kuriakose MI, D., & Migahed A. Assessment of tracheal intubating conditions in children using remifentanil and propofol without muscle relaxant. Pediatr Anesthesia. 2004;14:452–6.
Bulka CM, Terekhov MA, Martin BJ, Dmochowski RR, Hayes RM, Ehrenfeld JM. Nondepolarizing neuromuscular blocking agents, reversal, and risk of postoperative pneumonia. Anesthesiology. 2016;125:647–55.
Sneyd JR, O’Sullivan E. Tracheal intubation without neuromuscular blocking agents: is there any point? Br J Anaesth. 2010;104:535–7.
Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007;62:1266–80.
Egan TD. Remifentanil by bolus injection: a safety, pharmacokinetic, pharmacodynamic, and age effect investigation in human volunteers. Br J Anaesth. 2004;92:335–43.
Viby-Mogensen J, Engbaek J, Eriksson LI, Gramstad L, Jensen E, Jensen FS, Koscielniak-Nielsen Z, Skovgaard LT, Østergaard D. Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents. Acta Anaesthesiol Scand. 1996;40:59–74.
Dixon WJ. The up-and-down method for small samples. J Am Stat Assoc. 1965;60:967.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb S, Dixon-Woods M, McCulloch P, Wyatt J, Phelan AC, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Bmj. 2014;348:g1687.
Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology. Anesthesiology. 2007;107:144–52.
Klemola U-M, Hiller A. Tracheal intubation after induction of anesthesia in children with propofol—remifentanil or propofol-rocuronium. Can J Anesthesia. 2000;47:854–9.
Grant S, Noble S, Woods A, Murdoch J, Davidson A. Assessment of intubating conditions in adults after induction with propofol and varying doses of remifentanil. Br J Anaesth. 1998;81:540–3.
Bae JY, Kwak TY, Kim JW, Woo CH, Kim K-M. Tracheal intubation without the use of muscle relaxant in severe burn patients using propofol and varying doses of remifentanil. Korean J Anesthesiol. 2009;57:26.
Alexander R, Olufolabi AJ, Booth J, El-Moalem HE, Glass PS. Dosing study of remifentanil and propofol for tracheal intubation without the use of muscle relaxants. Anaesthesia. 1999;54:1037–40.
McNeil IA, Culbert B, Russell I. Comparison of intubating conditions following propofol and succinylcholine with propofol and remifentanil 2 micrograms kg-1 or 4 micrograms kg-1. Br J Anaesth. 2000;85:623–5.
Woods AW, Grant S, Harten J, Noble JS, Davidson JA. Tracheal intubating conditions after induction with propofol, remifentanil and lignocaine. Eur J Anaesthesiol. 1998;15:714–8.
Min SK, Kwak YL, Park SY, Kim JS, Kim JY. The optimal dose of remifentanil for intubation during sevoflurane induction without neuromuscular blockade in children. Anaesthesia. 2007;62:446–50.
Durmus M, Ender G, Kadir BA, Nurcin G, Erdogan O, Ersoy MO. Remifentanil with thiopental for tracheal intubation without muscle relaxants. Anesth Analg. 2003;96, 1336–9, (table of contents (2003)).
Sanford TJ, Weinger MB, Smith NT, Benthuysen JL, Head N, Silver H, Blasco TA. Pretreatment with sedative-hypnotics, but not with nondepolarizing muscle relaxants, attenuates alfentanil-induced muscle rigidity. J Clin Anesth. 1994;6:473–80.
Acknowledgements
We thank Professor Nathan L. Pace, from Utah University, who gently sent us R source codes for helping us analyzing our data.
Funding
The research was carried without funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Gabriel Magalhães Nunes Guimarães received funds from Pfizer in 2017 for writing a review about COX-2 drugs. The other authors declare no significant conflicts of interest.
Disclosures
Registry name: Clinical Trials. Date the trial was registered: May, 27, 2015. Date the first participant was enrolled: July, 4, 2016. URL of the registry: https://clinicaltrials.gov/ct2/show/NCT02454868. Trial registration number: NCT02454868. Trial registration name: Remifentanil Minimum Effective Dose for Children Intubation Without Neuromuscular Blockade.
About this article
Cite this article
Ono, A.H., Moura, T.R., Govêia, C.S. et al. ED50 of remifentanil for providing excellent intubating conditions when co-administered with a single standard dose of propofol without the use of muscle relaxants in children: dose-finding clinical trial. J Anesth 32, 493–498 (2018). https://doi.org/10.1007/s00540-018-2502-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-018-2502-z