Abstract
Background
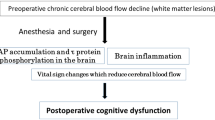
Postoperative cognitive dysfunction (POCD) is likely to occur in elderly people, who often suffer from cerebral hypoperfusion and white matter lesions even in the absence of cerebral infarctions.
Methods
Thirty-two adult male rats were randomly assigned to one of four groups: the cerebral normoperfusion + normotension group (n = 8), cerebral normoperfusion + hypotension group (n = 8), chronic cerebral hypoperfusion (CCH) + normotension group (n = 8), and CCH + hypotension group (n = 8). A rat model of CCH was developed via the permanent ligation of the bilateral common carotid arteries, but ligation was avoided in the cerebral normoperfusion groups. Two weeks later, the rats were intubated and mechanically ventilated under isoflurane anesthesia, and their mean arterial blood pressure was maintained over 80 mmHg (normotension) or below 60 mmHg (hypotension) for 2 h. After preparing brain slices, histological cresyl violet staining, ionized calcium binding adaptor molecule 1, a marker of microglial activation, or β amyloid precursor protein, a marker of axonal damage, were performed.
Results and conclusion
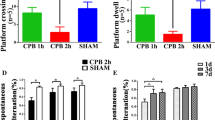
CCH per se caused microglial activation and axonal damage, which was not accentuated by hypotension. CCH alone did not cause neuronal damage, but CCH combined with hypotension caused significant neuronal damage in the hippocampal CA1 region. These results suggest that persistent hypotension during general anesthesia might cause neuronal damage in patients with CCH, such as elderly people, and contribute to prevention against POCD.



Similar content being viewed by others
References
Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet. 1998;351:857–61.
Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30.
Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, ISPOCD Group. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55.
Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–27.
Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z. The common anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein. Arch Neurol. 2009;66:620–31.
Zhang B, Dong Y, Zhang G, Moir RD, Xia W, Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE, Xie Z. The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein level under hypoxic conditions. J Biol Chem. 2008;283:11866–75.
Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alfa, IL-6, IL-1beta. Neurobiol Aging. 2012;33:1364–78.
Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M. Resolving postoperative neuroinflammation and cognitive decline. ANN Neurol. 2011;70:986–95.
Pantoni L, Garcia JH. Pathogenesis of leucoaraiosis. Stroke. 1997;28:652–9.
Nakao S, Xu Y. White matter injury in global cerebral ischemia. In: Carmichael Jr S, Baltan S, editors. White matter injury in stroke and CNS disease. New York: Springer; 2014. p. 181–96.
Maekawa K, Baba T, Otomo S, Morishita S, Tamura N. Low pre-existing gray matter volume in the medial temporal lobe and white matter lesions are associated with postoperative cognitive dysfunction after cardiac surgery. PLoS One. 2014. https://doi.org/10.1371/0087375.
Miyamoto E, Tomimoto H, Nakao S, Wakita H, Akiguchi I, Miyamoto K, Shingu K. Caudoputamen is damaged by hypocapnia during mechanical ventilation in a rat model of chronic cerebral hypoperfusion. Stroke. 2001;32:2920–5.
Miyamoto E, Nakao S, Tomimoto H, Wakita H, Yamada M, Masuzawa M, Takahira K, Sakamoto S, Shingu K. Ketamine attenuates hypoxia-induced neuronal damage in the caudoputamen in a rat model of chronic cerebral hypoperfusion. Neurosci Lett. 2004;354:26–9.
Ihara M, Tomimoto H, Kinoshita M, Oh J, Noda M, Wakita H, Akiguchi I, Shibasaki H. Chronic cerebral hypoperfusion induces MMP-2 but not MMP-9 expression in the microglia and vascular endothelium of the white matter. J Cereb Blood Flow Metab. 2001;21:828–34.
Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–94.
Kubo K, Nakao S, Jomura S, Sakamoto S, Miyamoto E, Xu Y, Tomimoto H, Inada T, Shingu K. Edaravone, a free radical scavenger, mitigates both gray and white matter damages after global ischemia in rats. Brain Res. 2009;1279:139–46.
Manso Y, Holland PR, Kitamura A, Szymkowiak S, Duncombe J, Hennessy E, Searcy JL, Marangoni M, Randall AD, Brown JT, McColl BW, Horsburgh K. Minocycline reduces microgliosis improves subcortical white matter function in a model of cerebral vascular disease. Glia. 2018;66:34–46.
Wang Y, Cong Y, Li J, Li X, Li B, Qi S. Comparison of invasive blood pressure measurements from the caudal ventral artery and femoral artery in male adult SD and Wister rats. PLoS One. 2013. https://doi.org/10.1371/0060625.
Zou C, Hao L, Tian H, Song C, Zhang Y, Zhou H, Liu L. The effect of sympathetic denervation on cerebral arteriogenesis after chronic cerebral hypoperfusion. Am J Med Sci. 2016;351:616–22.
Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, Lu Z. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–7.
Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalation anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Metab. 2006;27:1108–28.
Schallner N, Ulbrich F, Engelstaedter H, Biermann J, Auwaerter V, Loop T, Goebel U. Isoflurane but not sevoflurane or desflurane aggravates injury to neurons in vitro and vivo via p75NTR-NF-kB activation. Anesth Analg. 2014;119:1429–41.
Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–87.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamamoto, T., Iwamoto, T., Kimura, S. et al. Persistent isoflurane-induced hypotension causes hippocampal neuronal damage in a rat model of chronic cerebral hypoperfusion. J Anesth 32, 182–188 (2018). https://doi.org/10.1007/s00540-018-2458-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-018-2458-z