Abstract
Objective
We wished to evaluate the effect of sufentanil lipid nanoparticles on peripheral analgesia of inflammatory pain model rats.
Methods
Ninety SD rats were randomly divided into an inflammatory model group (group A, n = 54) and a blank control group (group B, n = 36). Group A was further divided into the sufentanil lipid nanoparticles group (group A1, n = 18), the sufentanil group (group A2, n = 18), and the inflammatory pain model group (group A3, n = 18); group B was divided into the sufentanil lipid nanoparticles group (group B1, n = 18) and the sufentanil group (group B2, n = 18). Rats of group A were given a formalin injection in the foot to produce the inflammatory pain model. Group B rats were given a normal saline foot injection of the same dosage. Then, groups A1 and B1 were given sufentanil lipid nanoparticles (0.82 μg/kg) treatment. Groups A2 and B2 were given sufentanil of the same dosage, and group A3 were given normal saline. Pain scores of Group A rats were recorded and analyzed. The ELISA method was adopted to determine drug concentration in rat brain, plasma, and the inflammatory pain/subcutaneous area.
Results
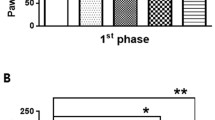
Pain scores of rats in group A3 were always higher than those in groups A1 and A2, and the pain scores of group A2 were higher than in group A1 0–30 min after administration (P < 0.05). The brain drug concentration in groups A2 and B1 fluctuated over time; the brain drug concentrations of groups A2 and B2 were respectively higher than those of groups A1 and B1 (P < 0.05). There was no significant difference between the plasma drug concentrations of different groups at the same time point (P > 0.05); however, there was a notable difference within each group at different time points (P < 0.05), and the drug concentration of the inflammatory tissues in group A1 changed significantly over time (P < 0.05). Thirty minutes after administration, drug concentration in the inflammatory site of group A1 was higher than that of groups A2, B1, and B2 (P < 0.05).
Conclusion
Sufentanil lipid nanoparticles had a comparatively weak effect on the central nervous system because of their features such as large particle size and targeted and controlled release. They have shown a remarkable analgesic effect in the peripheral inflammatory pain areas.


Similar content being viewed by others
References
Gebhart GF, Su X, Joshi S, Ozaki N, Sengupta JN. Peripheral opioid modulation of visceral pain. Ann N Y Acad Sci. 2000;909:41–50.
Dabbagh A, Rajaei S. Halothane: is there still any place for using the gas as an anesthetic? Hepat Mon. 2011;11(7):511–2.
Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–15.
Korotcov A, Shan L, Meng H, Wang T, Sridhar R, Zhao Y, Liang XJ, Wang PC. A nanocomplex system as targeted contrast agent delivery vehicle for magnetic resonance imaging dynamic contrast enhancement study. J Nanosci Nanotechnol. 2010;10(11):7545–9.
de Araújo DR, da Silva DC, Barbosa RM, Franz-Montan M, Cereda CM, Padula C, Santi P, de Paula E. Strategies for delivering local anesthetics to the skin: focus on liposomes, solid lipid nanoparticles, hydrogels and patches. Expert Opin Drug Deliv. 2013;10:1551–63.
Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–74.
Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14(3):249–58.
Brack A, Rittner HL, Machelska H, Beschmann K, Sitte N, Schäfer M, Stein C. Mobilization of opioid containing polymorphonuclear cells by hematopoietic growth factors and influence on inflammatory pain. Anesthesiology. 2004;100:149–57.
Andreozzi E, Wang P, Valenzuela A, Tu C, Gorin F, Dhenain M, Louie A. Size-stable solid lipid nanoparticles loaded with Gd-DOTA for magnetic resonance imaging. Bioconjug Chem. 2013;24:1455–67.
Pathak P, Nagarsenker M. Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech. 2009;10(3):985–92.
Leng F, Wan J, Liu W, Tao B, Chen X. Prolongation of epidural analgesia using solid lipid nanoparticles as drug carrier for lidocaine. Reg Anesth Pain Med. 2012;37(2):159–65.
Bocca C, Caputo O, Cavalli R, Gabriel L, Miglietta A, Gasco MR. Phagocytic uptake of fluorescent stealth and non-stealth solid lipid nanoparticles. Int J Pharm 1998;175(2):185–93.
Cavalli R, Bocca C, Miglietta A, Caputo O, Gasco MR. Albumin adsorption on stealth and non-stealth solid lipid nanoparticles. STP Pharm Sci 1999;9(2):183–9.
Guan QX, Zhu K, Lin TM, Guan QT, Guo J, Yin JY. Preparation, characterization and drug release of biodegradable solid lipid nanoparticles. Chem J Chin Univ. 2010;31(11):2298–302.
Jigisha KV, Shamsunder SD, Krutika KS. Cyclosporine A loaded solid lipid nanoparticles: optimization of formulation process variable and characterization. Curr Drug Deliv. 2008;5:64.
Zhang XX, Pan WS, Gan L, Zhu C, Gan Y, Nie S. Preparation of a dispersible PEGylate nanostructured lipid carrier (NLC) loaded with 10-hydroxycamptothecin by spray-drying. Chem Pharm Bull. 2008;56:1645–50.
Zhang L, Zhang N. How nanotechnology can enhance docetaxel therapy. Int J Nanomed. 2013;8:2927–41.
Acknowledgments
The accomplishment of this dissertation is the joint efforts of all colleagues in the hospital. First, workmates in the department have offered great help in the construction of the dissertation. Second, the hospital offered the necessary experimental instruments. Thus, sincere gratitude is given to them.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, H., Qiao, H., Lu, H. et al. Evaluation of the peripheral analgesic effect of sufentanil lipid nanoparticles . J Anesth 28, 702–707 (2014). https://doi.org/10.1007/s00540-014-1795-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-014-1795-9