Abstract
Purpose
The purpose of the present study was to investigate whether thrombomodulin (TM) prevents the development of pulmonary hypertension (PH) in monocrotaline (MCT)-injected rats.
Methods
Human recombinant TM (3 mg/kg/2 days) or saline were given to MCT-injected male Sprague–Dawley rats for 19 (n = 14) or 29 (n = 11) days. Control rats (n = 6) were run for 19 days. The mean pulmonary artery pressure (mPAP), right ventricular hypertrophy (RVH), percentages of muscularized peripheral arteries (%muscularization), and medial wall thickness of small muscular arteries (%MWT) were measured. To determine inflammatory and coagulation responses, broncho-alveolar lavage fluid (BALF) was analyzed in another set of rats (n = 29). Western blotting for endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS (peNOS) in the lung tissue was performed in separate rats (n = 13). Survival was determined in 60 rats.
Results
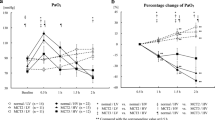
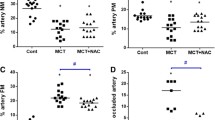
MCT increased mPAP, RVH, %muscularization, and %MWT. TM treatment significantly reduced mPAP, %muscularization, and %MWT in peripheral arteries with an external diameter of 50–100 μm in 19 days after MCT injection, but the effect was lost after 29 days. MCT increased the levels of tumor necrosis factor alpha, monocyte chemoattractant protein-1, and thrombin-antithrombin complex in BALF. Expression of eNOS increased in MCT rats, while peNOS decreased. The relative amount of peNOS to total eNOS increased in MCT/TM rats compared to MCT/Vehicle rats. A Kaplan–Meier survival curve showed no difference with and without TM.
Conclusion
Although the administration of TM might slightly delay the progression of MCT-induced PH, the physiological significance for treatment is limited, since the survival rate was not improved.






Similar content being viewed by others
References
Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118:2372–9.
Mitani Y, Maruyama K, Sakurai M. Prolonged administration of l-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation. 1997;96:689–769.
Maruyama J, Maruyama K, Mitani Y, Kitabatake M, Yamauchi T, Miyasaka K. Continuous low-dose NO inhalation does not prevent monocrotaline-induced pulmonary hypertension in rats. Am J Physiol. 1997;272:H517–24.
Zhang E, Jiang B, Yokochi A, Maruyama J, Mitani Y, Ma N, Maruyama K. Effect of all-trans-retinoic acid on the development of chronic hypoxia-induced pulmonary hypertension. Circ J. 2010;74:1696–703.
Jiang BH, Maruyama J, Yokochi A, Iwasaki M, Amano H, Mitani Y, Maruyama K. Prolonged nitric oxide inhalation fails to regress hypoxic vascular remodeling in rat lung. Chest. 2004;125:2247–52.
Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–63.
Maruyama K, Maruyama J, Utsunomiya H, Furuhashi K, Kurobuchi M, Katayama Y, Yada I, Muneyuki M. Effect of nicardipine on pulmonary hypertension after repair of congenital heart defects in early postoperative period. J Anesth. 1993;7:95–101.
Maruyama K, Nakai Y, Chikusa H, Muneyuki M. Verapamil reduced pulmonary hypertension in adult respiratory distress syndrome. J Anesth. 1994;8:480–1.
Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, Mason RJ. Overexpression of tumor necrosis factor-α produces an increase in lung volume and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;280:L39–49.
Miyata M, Ito M, Sasajima T, Ohira H, Kasukawa R. Effect of a serotonin receptor antagonist on interleukin-6-induced pulmonary hypertension in rats. Chest. 2001;119:554–61.
Stenmark KR, Morganroth ML, Remigio LK, Voelkel NF, Murphy RC, Henson PM, Mathias MM, Reeves JT. Alveolar inflammation and arachidonate metabolism in monocrotaline-induced pulmonary hypertension. Am J Physiol. 1985;248:H856–66.
Zapol WM, Jones R. Vascular components of ARDS. Clinical pulmonary hemodynamics and morphology. Am Rev Respir Dis. 1987;136:471–4.
Snow RL, Davies P, Pontoppidan H, Zapol WM, Reid L. Pulmonary vascular remodeling in adult respiratory distress syndrome. Am Rev Respir Dis. 1982;126:887–92.
Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54.
Saito H, Maruyama S, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M, Aoki N. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in deisseminated intravascular coagulation: results of a phase III, randomized, double-blinded clinical trial. J Thromb Haemost. 2007;5:31–41.
Iba T, Nagaoka I, Boulat M. The anticoagulant therapy for sepsis-associated disseminated intravascular coagulation. Thromb Res. 2013 in press.
Weiler H, Isermann BH. Thrombomodulin. J Thromb Haemost. 2003;1:1515–24.
Van de Wouwer M, Conway EM. Novel functions of thrombomodulin in inflammation. Crit Care Med. 2004;32:S254–61.
Grinnell BW, Berg DT. Surface thrombomodulin modulates thrombin receptor responses on vascular smooth muscle cell. Am J Physiol. 1996;270:H603–9.
Shi CS, Shi GY, Chang YS, Han HS, Kuo CH, Liu C, Huang HC, Chang YJ, Chen PS, Wu HL. Evidence of human thrombomodulin domain as a novel angiogenic factor. Circulation. 2005;111:1627–36.
David-Dufilho M, Brussel EMV, Topal G, Walch L, Brunet A, Rendu F. Endothelial thrombomodulin induces Ca2+ signals and nitric oxide synthesis through epidermal growth factor receptor kinase and calmodulin kinase II. J Biol Chem. 2005;280:35999–6006.
Ikeguchi H, Maruyama S, Morita Y, Fujita Y, Kato T, Natori Y, Akastu H, Campbell W, Okada N, Okada H, Yuzawa Y, Mastuo S. Effects of human soluble thrombomodulin on experimental glomerulonephritis. Kid Int. 2002;61:490–501.
Nagato M, Okamoto K, Abe Y, Higure A, Yamaguchi K. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-a protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit Care Med. 2009;37:2181–6.
Roberts JD Jr, Roberts CT, Jones RC, Zapol WM, Bloch KD. Continuous nitric oxide inhalation reduces pulmonary arterial structural changes, right ventricular hypertrophy, and growth retardation in the hypoxic newborn rat. Circ Res. 1995;76:215–22.
Kimura H, Kasahara Y, Kurosu K, Sugito K, Takiguchi Y, Terai M, Mikata A, Natsume M, Mukaida N, Matsushima K, Kuriyama T. Alleviation of monocrotaline-induced pulmonary hypertension by antibodies to monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest. 1998;78:571–81.
Chang LT, Sun CK, Sheu JJ, Chiang CH, Youssef AA, Lee FY, Wu CJ, Yip HK. Cilostazol therapy attenuates monocrotaline-induced pulmonary arterial hypertension in rat model. Circ J. 2008;72:825–31.
Sawada H, Mitani Y, Maruyama J, Jiang BH, Ikeyama Y, Dida FA, Yamamoto H, Imanaka-Yoshida K, Shimpo H, Mizoguchi A, Maruyama K, Komada Y. A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest. 2007;132:1265–74.
Wang FM, Wang DW, Yang SW. Changes of thrombomodulin in rats with pulmonary hypertension induced by monocrotaline. Zhonghua Er Ke Za Zhi. 2007;45:297–8.
Moll S, Lindley C, Pescatore S, Morrison D, Tsuruta K, Mohri M, Serada M, Sata M, Shimizu H, Yamada K, White GC II. Phase I study of a novel recombinant human soluble thrombomodulin, ART-123. J Thromb Haemost. 2004;2:1745–51.
Cacoub P, Karmochkine M, Dorent R, Nataf P, Piette JC, Godeau P, Gandjbakhch IG, Boffa MC. Plasma levels of thrombomodulin in pulmonary hypertension. Am J Med. 1996;101:160–4.
Sakamaki F, Kyotani S, Nagaya N, Sato N, Oya H, Satoh T, Nakanishi N. Increased plasma p-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation. 2000;102:2720–5.
Ventetuolo CE, Klinger JR. WHO Group 1 pulmonary arterial hypertension: current and investigative therapies. Prog Cardiovasc Dis. 2012;55:89–103.
Strieter RM, Kunkel SL. The immunopathology of chemotactic cytokines. Adv Exp Med Biol. 1993;351:19–28.
Ikeda Y, Yonemitsu Y, Kataoka C, Kitamoto S, Yamaoka T, Nishida K, Takeshita A, Egashira K, Sueishi K. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2002;283:H2021–8.
Jin H, Yang X, Liu K, Gu Q, Xu X. Effects of a novel peptide derived from human thrombomodulin on endotoxin-induced uveitis in vitro and in vivo. FEBS Lett. 2011;585:3457–64.
Nishii Y, Gabazza EC, Fujimoto H, Nakahara H, Takagi T, Bruno N, D’Alessandro-Gabazza CN, Maruyama J, Maruyama K, Hayashi T, Adachi Y, Suzuki K, Taguchi O. Protective role of protein C inhibitor in monocrotaline-induced pulmonary hypertension. J Thromb Haemost. 2006;4:2331–9.
Suzuki K, Kusumoto H, Deyashiki Y, Nishioka J, Maruyama I, Zushi M, Kawahara S, Honda G, Yamamoto S, Horiguchi S. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987;6:1891–7.
Acknowledgments
This work was supported in part by Grants-In-Aid for Scientific Research 16390449, 20590920, and 21592003 from the Japanese Ministry of Education, Science and Culture.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamada, Y., Maruyama, J., Zhang, E. et al. Effect of thrombomodulin on the development of monocrotaline-induced pulmonary hypertension. J Anesth 28, 26–33 (2014). https://doi.org/10.1007/s00540-013-1663-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-013-1663-z