Abstract
Purpose
Recent evidence has indicated that post-tetanic motor evoked potentials (p-MEPs) can be used to improve the reliability of the monitoring of motor function during spinal surgery. However, data on p-MEP monitoring are limited to those in subjects under propofol anesthesia. The present study was conducted to assess the applicability of sevoflurane during p-MEP monitoring in patients undergoing spinal surgery.
Methods
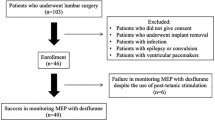
Thirty-five patients undergoing spinal surgery under sevoflurane anesthesia were enrolled in the study and classified as being without preoperative motor deficits (n = 25) or with preoperative motor deficits (n = 10). For conventional MEP (c-MEP), transcranial train-pulse stimulation was delivered and the compound muscle action potentials were bilaterally recorded from the abductor pollicis brevis, abductor hallucis, tibialis anterior, and soleus muscles. For p-MEP, tetanic stimulation (50 Hz, 50 mA stimulus intensity) for 5 s was applied to the bilateral median and left tibial nerves 1 s prior to transcranial stimulation.
Results
The amplitudes of p-MEP were significantly higher in all muscle recording sites than those of c-MEP in patients without motor deficits, whereas these amplitudes were significantly higher in only four of the eight muscles in patients with motor deficits (P < 0.05). The success rates of c-MEP and p-MEP recording were 48% and 64%, respectively, in patients without motor deficits and 30% and 60%, respectively, in patients with motor deficits. There were no statistically significant differences in success rates between c-MEP and p-MEP recording.
Conclusion
Although the application of tetanic stimulation prior to transcranial stimulation did not significantly increase the success rates of MEP recording, it significantly enlarged MEP amplitude under sevoflurane anesthesia in patients without preoperative motor deficits.
Similar content being viewed by others
References
Kawaguchi M, Furuya H. Intraoperative spinal cord monitoring of motor function with myogenic motor evoked potentials: a consideration in anesthesia. J Anesth. 2004;18:18–28.
Kalkman CJ, Been HD, Ongerboer de Visser BW. Intraoperative monitoring of spinal cord function. A review. Acta Orthop Scand. 1993;64:114–123.
Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:430–443.
Lotto ML, Banoub M, Schubert A. Effects of anesthetic agents and physiologic changes on intraoperative motor evoked potentials. J Neurosurg Anesthesiol. 2004;16:32–42.
Kawaguchi M, Shimizu K, Furuya H, Sakamoto T, Ohnishi H, Karasawa J. Effect of isoflurane on motor-evoked potentials induced by direct electrical stimulation of the exposed motor cortex with single, double, and triple stimuli in rats. Anesthesiology. 1996;85:1176–1183.
Kawaguchi M, Inoue S, Kakimoto M, Kitaguchi K, Furuya H, Morimoto T, Sakaki T. The effect of sevoflurane on myogenic motor-evoked potentials induced by single and paired transcranial electrical stimulation of the motor cortex during nitrous oxide/ketamine/fentanyl anesthesia. J Neurosurg Anesthesiol. 1998;10:131–136.
Kawaguchi M, Sakamoto T, Inoue S, Kakimoto M, Furuya H, Morimoto T, Sakaki T. Low dose propofol as a supplement to ketamine-based anesthesia during intraoperative monitoring of motor-evoked potentials. Spine. 2000;25:974–979.
Yamamoto Y, Kawaguchi M, Kakimoto M, Takahashi M, Inoue S, Goto T, Furuya H. The effects of xenon on myogenic motor evoked potentials in rabbits: a comparison with propofol and isoflurane. Anesth Analg. 2006;102:1715–1721.
Yamamoto Y, Kawaguchi M, Kakimoto M, Inoue S, Furuya H. The effects of dexmedetomidine on myogenic motor evoked potentials in rabbits. Anesth Analg. 2007;104:1488–1492.
Ubags LH, Kalkman CJ, Been HD. Influence of isoflurane on myogenic motor evoked potentials to single and multiple transcranial stimuli during nitrous oxide/opioid anesthesia. Neurosurgery. 1998;43:90–94.
Zentner J, Albrecht T, Heuser D. Influence of halothane, enflurane, and isoflurane on motor evoked potentials. Neurosurgery. 1992;31:298–305.
Scheufler KM, Zentner J. Total intravenous anesthesia for intraoperative monitoring of the motor pathways: an integral view combining clinical and experimental data. J Neurosurg. 2002;96:571–579.
Kalkman CJ, Drummond JC, Ribberink AA, Patel PM, Sano T, Bickford RG. Effects of propofol, etomidate, midazolam, and fentanyl on motor evoked responses to transcranial electrical or magnetic stimulation in humans. Anesthesiology. 1992;76:502–509.
Kakimoto M, Kawaguchi M, Yamamoto Y, Inoue S, Horiuchi T, Nakase H, Sakaki T, Furuya H. Tetanic stimulation of the peripheral nerve before transcranial electrical stimulation can enlarge general anesthesia with neuromuscular blockade. Anesthesiology. 2005;102:733–738.
Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H. The application of tetanic stimulation of the unilateral tibial nerve before transcranial stimulation can augment the amplitudes of myogenic motor-evoked potentials from the muscles in the bilateral upper and lower limbs. Anesth Analg. 2008;107:215–220.
Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H. Evaluation of reliability of post-tetanic motor evoked potential monitoring during spinal surgery under general anesthesia. Spine. 2008;33:E994–E1000.
Kawaguchi M, Hayashi H, Yamamoto Y, Furuya H. Recent advances in the monitoring of myogenic motor-evoked potentials: development of post-tetanic motor-evoked potentials. J Anesth. 2008;22:489–492.
Ali HH, Savarese JJ. Monitoring of neuromuscular function. Anesthesiology. 1976;45:216–249.
Wali FA, Bradshaw EG, Suer AH. Clinical assessment of neuromuscular blockade produced by vecuronium using twitch, train of four tetanus and post-tetanic twitch response of the adductor pollicis muscle. Acta Anaesth Belg. 1988;39:35–42.
Ridley SA, Hatch DJ. Post-tetanic count and profound neuromuscular blockade with atracurium infusion in paediatric patients. Br J Anaesth. 1988;60:31–35.
Gwinnutt CL, Meakin G. Use of the post-tetanic count to monitor recovery from intense neuromuscular blockade in children. Br J Anaesth. 1988;61:547–550.
Saitoh Y, Narumi Y, Fujii Y. Post-tetanic count and train-of-four responses during neuromuscular block produced by vecuronium and infusion of nicardipine. Br J Anaesth. 1999;83:340–342.
Luft AR, Kaelin-Lang A, Hauser TK, Buitrago MM, Thakor NV, Hanley DF, Cohen LG. Modulation of rodent cortical motor excitability by somatosensory input. Exp Brain Res. 2002;142:562–569.
Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633.
Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1:64–68.
Andersson G, Ohlin A. Spatial facilitation of motor evoked responses in monitoring during spinal surgery. Clin Neurophysiol. 1999;110:720–724.
Reinacher PC, Priebe HJ, Blumrich W, Zentner J, Scheufler KM. The effects of stimulation pattern and sevoflurane concentration on intraoperative motor-evoked potentials. Anesth Analg. 2006;102:888–895.
Author information
Authors and Affiliations
About this article
Cite this article
Hayashi, H., Kawaguchi, M., Abe, R. et al. Evaluation of the applicability of sevoflurane during post-tetanic myogenic motor evoked potential monitoring in patients undergoing spinal surgery. J Anesth 23, 175–181 (2009). https://doi.org/10.1007/s00540-008-0733-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-008-0733-0