Abstract
Background
Information on the dynamics of metabolic dysfunction-associated steatotic liver disease (MASLD) among hepatitis C virus patients achieving sustained virologic response (SVR12) with direct-acting antivirals (DAAs) is limited.
Methods
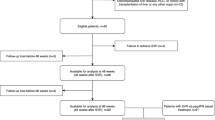
We enrolled 1512 eligible participants in this prospective study. MASLD was defined by a controlled attenuation parameter (CAP) of ≥248 dB/m utilizing vibration-controlled transient elastography in conjunction with presence of ≥1 cardiometabolic risk factor. The distribution of MASLD and the changes in CAP were evaluated before treatment and at SVR12. Forward stepwise logistic regression analyses were performed to determine factors significantly associated with the regression or emergence of MASLD.
Results
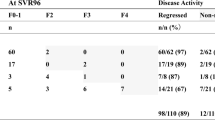
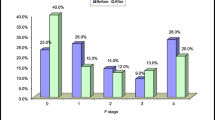
The prevalence of MASLD decreased from 45.0% before treatment to 36.1% at SVR12. Among 681 participants with MASLD before treatment, 144 (21%) exhibited MASLD regression at SVR12. Conversely, among 831 participants without MASLD before treatment, 9 (1.1%) developed MASLD at SVR12. Absence of type 2 diabetes (T2D) [odds ratio (OR): 1.73, 95% confidence interval (CI): 1.13–2.65, p = 0.011], age > 50 years (OR: 1.73, 95% CI: 1.11–2.68, p = 0.015), and alanine transaminase (ALT) ≤ 2 times the upper limit of normal (ULN) (OR: 1.56; 95% CI: 1.03–2.37, p = 0.035) were associated with the regression of MASLD. Presence of T2D was associated with the emergence of MASLD (OR: 5.83, 95% CI: 1.51–22.56, p = 0.011).
Conclusions
The prevalence of MASLD decreased after achieving SVR12 with DAAs. Patients with pre-existing T2D showed a diminished probability of MASLD regression and a heightened risk of MASLD emergence post-SVR12.




Similar content being viewed by others
Abbreviations
- DAA:
-
Direct-acting antiviral
- HCV:
-
Hepatitis C virus
- SVR12 :
-
Sustained virologic response
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- IR:
-
Insulin resistance
- T2D:
-
Type 2 diabetes
- HCC:
-
Hepatocellular carcinoma
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- MASH:
-
Metabolic dysfunction-associated steatohepatitis
- MS:
-
Metabolic syndrome
- NIT:
-
Noninvasive test
- VCTE:
-
Vibration-controlled transient elastography
- CAP:
-
Controlled attenuation parameter
- MRI:
-
Magnetic resonance imaging
- PDFF:
-
Proton density fat fraction
- IFN:
-
Interferon
- RNA:
-
Ribonucleic acid
- LLOQ:
-
Lower limit of quantification
- dB:
-
Decibel
- CMRF:
-
Cardiometabolic risk factor
- BMI:
-
Body mass index
- HbA1c:
-
Glycosylated hemoglobin
- HTN:
-
Hypertension
- HDL-C:
-
High-density lipoprotein-cholesterol
- HBV:
-
Hepatitis B virus
- HIV:
-
Human immunodeficiency virus
- LSM:
-
Liver stiffness measurement
- SLD:
-
Steatotic liver disease
- INR:
-
International normalized ratio
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- FIB-4:
-
Fibrosis index based on four parameters
- HBsAg:
-
Hepatitis B surface antigen
- kPa:
-
Kilo Pascal
- ULN:
-
Upper limit of normal
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SREBP:
-
Sterol regulatory element-binding protein
- LDL-C:
-
Low-density lipoprotein-cholesterol
- VLDL-C:
-
Very low-density lipoprotein-cholesterol
- MTP:
-
Microsomal triglyceride transport protein
References
Martinello M, Solomon SS, Terrault NA, et al. Hepatitis C. Lancet. 2023;402:1085–96.
Negro F. Residual risk of liver disease after hepatitis C virus eradication. J Hepatol. 2021;74:952–63.
Younossi ZM, Golabi P, Paik JM, et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–47.
Cacoub P, Saadoun D. Extrahepatic manifestations of chronic HCV infection. N Engl J Med. 2021;384:1038–52.
Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24.
Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521–30.
Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691–705.
Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. 2022;71:156–62.
Dyal HK, Aguilar M, Bhuket T, et al. Concurrent obesity, diabetes, and steatosis increase risk of advanced fibrosis among HCV patients: a systematic review. Dig Dis Sci. 2015;60:2813–24.
Dyal HK, Aguilar M, Bartos G, et al. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. 2016;61:636–45.
Peleg N, Issachar A, Sneh Arbib O, et al. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J Viral Hepat. 2019;26:1257–65.
van der Meer AJ, Feld JJ, Hofer H, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66:485–93.
Benhammou JN, Moon AM, Pisegna JR, et al. Nonalcoholic fatty liver disease risk factors affect liver-related outcomes after direct-acting antiviral treatment for hepatitis C. Dig Dis Sci. 2021;66:2394–406.
Degasperi E, D’Ambrosio R, Iavarone M, et al. Factors associated with increased risk of de novo or recurrent hepatocellular carcinoma in patients with cirrhosis treated with direct-acting antivirals for HCV infection. Clin Gastroenterol Hepatol. 2019;17:1183-91.e7.
Wiese M, Fischer J, Löbermann M, et al. Evaluation of liver disease progression in the German hepatitis C virus (1b)-contaminated anti-D cohort at 35 years after infection. Hepatology. 2014;59:49–57.
Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;76:1542–56.
Fouad Y, Lazarus JV, Negro F, et al. MAFLD considerations as a part of the global hepatitis C elimination effort: an international perspective. Aliment Pharmacol Ther. 2021;53:1080–9.
Castéra L, Hézode C, Roudot-Thoraval F, et al. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53:420–4.
Shimizu K, Soroida Y, Sato M, et al. Eradication of hepatitis C virus is associated with the attenuation of steatosis as evaluated using a controlled attenuation parameter. Sci Rep. 2018;8:7845.
Tada T, Kumada T, Toyoda H, et al. Viral eradication reduces both liver stiffness and steatosis in patients with chronic hepatitis C virus infection who received direct-acting anti-viral therapy. Aliment Pharmacol Ther. 2018;47:1012–22.
Kobayashi N, Iijima H, Tada T, et al. Changes in liver stiffness and steatosis among patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. Eur J Gastroenterol Hepatol. 2018;30:546–51.
Niu B, Zang W, Zhou H, et al. Regression in hepatic fibrosis in elderly Chinese patients with hepatitis C receiving direct-acting antiviral treatment. BMC Gastroenterol. 2023;23:102.
Chuaypen N, Siripongsakun S, Hiranrat P, et al. Improvement of liver fibrosis, but not steatosis, after HCV eradication as assessment by MR-based imaging: role of metabolic derangement and host genetic variants. PLoS ONE. 2022;17: e0269641.
Kawagishi N, Suda G, Nakamura A, et al. Liver steatosis and dyslipidemia after HCV eradication by direct acting antiviral agents are synergistic risks of atherosclerosis. PLoS ONE. 2018;13: e0209615.
Tokuchi Y, Suda G, Kawagishi N, et al. Hepatitis C virus eradication by direct-acting antivirals causes a simultaneous increase in the prevalence of fatty liver and hyper low-density lipoprotein cholesterolemia without an increase in body weight. Hepatol Res. 2023;53:595–606.
Trifan A, Stratina E, Rotaru A, et al. Changes in liver steatosis using controlled attenuation parameter among patients with chronic hepatitis C infection treated with direct-acting antivirals therapy who achieved sustained virological response. Diagnostics (Basel). 2022;12:702.
Rout G, Nayak B, Patel AH, et al. Therapy with oral directly acting agents in hepatitis C infection is associated with reduction in fibrosis and increase in hepatic steatosis on transient elastography. J Clin Exp Hepatol. 2019;9:207–14.
Graf C, Welzel T, Bogdanou D, et al. Hepatitis C clearance by direct-acting antivirals impacts glucose and lipid homeostasis. J Clin Med. 2020;9:2702.
Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30.
Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–35.
Wong VW, Petta S, Hiriart JB, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017;67:577–84.
Liu CH, Liang CC, Liu CJ, et al. Comparison of Abbott RealTime HCV genotype II with versant line probe assay 2.0 for hepatitis C virus genotyping. J Clin Microbiol. 2015;53:1754–7.
Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25.
Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–47.
Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10.
Ribaldone DG, Sacco M, Saracco GM. The effect of viral clearance achieved by direct-acting antiviral agents on hepatitis C virus positive patients with type 2 diabetes mellitus: a word of caution after the initial enthusiasm. J Clin Med. 2020;9:563.
Ciancio A, Ribaldone DG, Dotta A, et al. Long-term follow-up of diabetic and non-diabetic patients with chronic hepatitis C successfully treated with direct-acting antiviral agents. Liver Int. 2021;41:276–87.
Butt AA, Yan P, Aslam S, et al. Hepatitis C virus (HCV) treatment with directly acting agents reduces the risk of incident diabetes: results from electronically retrieved cohort of HCV infected veterans (ERCHIVES). Clin Infect Dis. 2020;70:1153–60.
Elgretli W, Chen T, Kronfli N, et al. Hepatitis C virus-lipid interplay: pathogenesis and clinical impact. Biomedicines. 2023;11:271.
Corey KE, Kane E, Munroe C, et al. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030–7.
Meissner EG, Lee YJ, Osinusi A, et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790–801.
Carvalho JR, Velosa J, Serejo F. Lipids, glucose and iron metabolic alterations in chronic hepatitis C after viral eradication - comparison of the new direct-acting antiviral agents with the old regimens. Scand J Gastroenterol. 2018;53:857–63.
McPherson S, Jonsson JR, Barrie HD, et al. Investigation of the role of SREBP-1c in the pathogenesis of HCV-related steatosis. J Hepatol. 2008;49:1046–54.
El-Ghandour A, Youssif T, Ibrahim W, et al. The effect of different direct antivirals on hepatic steatosis in nondiabetic and naïve hepatitis C-infected Egyptian patients. Egypt J Intern Med. 2023;35:12.
Mihai F, Trifan A, Stanciu C, et al. L3 skeletal muscle index dynamics in patients with HCV-related compensated cirrhosis following sustained virological response after direct acting antiviral treatment. Medicina (Kaunas). 2021;57:1226.
Sakamori R, Yamada R, Shinkai K, et al. Improvement of skeletal muscle mass after ledipasvir and sofosbuvir treatment for hepatitis C virus in decompensated liver cirrhosis. Intern Med. 2021;60:745–50.
Chadha N, Turner A, Sterling RK. Prevalence and predictors of abnormal alanine aminotransferase in patients with HCV who have achieved SVR. J Viral Hepat. 2023;30:73–8.
Olveira A, Domínguez L, Troya J, et al. Persistently altered liver test results in hepatitis C patients after sustained virological response with direct-acting antivirals. J Viral Hepat. 2018;25:818–24.
Acknowledgements
The authors thank Hui-Ju Lin and Pin-Chin Huang for clinical data management; the 7th Core Lab of the National Taiwan University Hospital, and the 1st Common Laboratory of the National Taiwan University Hospital, Yun-Lin Branch, for the instrumental and technical support.
Funding
The study was supported by National Science and Technology Council, Taiwan (NSTC 112-2314-B-002-131-MY3) and National Taiwan University Hospital (112-IF0004).
Author information
Authors and Affiliations
Contributions
Conceptualization: Chen-Hua Liu, Jia-Horng Kao; Data curation: Chen-Hua Liu; Formal analysis: Chen-Hua Liu, Yu-Ping Chang, Jia-Horng Kao; Funding acquisition: Chen-Hua Liu; Investigation: Chen-Hua Liu, Yu-Ping Chang, Yu-Jen Fang, Pin-Nan Cheng, Chi-Yi Chen, Wei-Yu Kao, Chih-Lin Lin, Sheng-Shun Yang, Yu-Lueng Shih, Cheng-Yuan Peng, Ming-Chang Tsai, Shang-Chin Huang, Tung-Hung Su, Tai-Chung Tseng, Chun-Jen Liu, Pei-Jer Chen, Jia-Horng Kao; Methodology: Chen-Hua Liu, Yu-Ping Chang; Project administration: Chen-Hua Liu, Yu-Ping Chang, Jia-Horng Kao; Resources: Chen-Hua Liu, Yu-Ping Chang, Yu-Jen Fang, Pin-Nan Cheng, Chi-Yi Chen, Wei-Yu Kao, Chih-Lin Lin, Sheng-Shun Yang, Yu-Lueng Shih, Cheng-Yuan Peng, Ming-Chang Tsai, Shang-Chin Huang, Tung-Hung Su, Tai-Chung Tseng, Chun-Jen Liu, Pei-Jer Chen, Jia-Horng Kao; Software: Chen-Hua Liu; Supervision: Jia-Horng Kao; Validation: Chen-Hua Liu, Yu-Ping Chang; Visualization: Chen-Hua Liu, Yu-Ping Chang; Writing – original draft: Chen-Hua Liu, Yu-Ping Chang, Jia-Horng Kao; Writing – review & editing: Chen-Hua Liu, Yu-Ping Chang, Yu-Jen Fang, Pin-Nan Cheng, Chi-Yi Chen, Wei-Yu Kao, Chih-Lin Lin, Sheng-Shun Yang, Yu-Lueng Shih, Cheng-Yuan Peng, Ming-Chang Tsai, Shang-Chin Huang, Tung-Hung Su, Tai-Chung Tseng, Chun-Jen Liu, Pei-Jer Chen, Jia-Horng Kao.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2024_2101_MOESM2_ESM.jpg
Supplementary Figure 1. Change in CAP level before treatment and at SVR12. The horizontal lines within the boxes are the median levels. The tops and bottoms of the boxes are the first and the third quartiles. The tops and the bottoms of the horizontal lines are the upper and lower whiskers. The circles denote mild outliers and the asterisks denote extreme outliers. The median levels of change in CAP from pre-treatment to SVR12 were (A) −8 dB/m (IQR: −14 to −2 dB/m) in the entire study population; (B) −8 dB/m (IQR: −15 to −3 dB/m) in participants with MASLD before treatment; (C) −8 dB/m (IQR: −13 to −2 dB/m) in participants without MASLD before treatment. CAP, controlled attenuation parameter, IQR, interquartile range; dB, decibel (JPG 381 KB)
535_2024_2101_MOESM3_ESM.jpg
Supplementary Figure 2. Change in CAP level among participants with different patterns of MASLD evolution before and after treatment. The horizontal lines within the boxes are the median levels. The tops and the bottoms of the boxes are the first and the third quartiles. The tops and the bottoms of the horizontal lines are the upper and the lower whiskers. The circles denote mild outliers and the asterisks denote extreme outliers. The median levels of change in CAP from pre-treatment to SVR12 were (A) −6 dB/m (IQR: −13 to −2 dB/m) in Group 1; (B) −16 dB/m (IQR: −32 to 11 dB/m) in Group 2; (C) −8 dB/m (IQR: −13 to −2 dB/m) in Group 3; (D) 31 dB/m (IQR: 7–64 dB/m) in Group 4. The p values were < 0.001 between Group 1 and Group 2, and between Group 3 and Group 4. CAP, controlled attenuation parameter, IQR, interquartile range; dB, decibel (JPG 54 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, CH., Chang, YP., Fang, YJ. et al. Dynamic change of metabolic dysfunction-associated steatotic liver disease in patients with hepatitis C virus infection after achieving sustained virologic response with direct-acting antivirals. J Gastroenterol (2024). https://doi.org/10.1007/s00535-024-02101-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00535-024-02101-2