Abstract
Background
Calcium voltage-gated channel auxiliary subunit alpha 2/delta 1 (CACNA2D1), a gene encoding a voltage-gated calcium channel, has been reported as an oncogene in several cancers. However, its role in colon cancer (CC) remains unclear. This study aimed to investigate the function of CACNA2D1 and its effect on the microenvironment in CC.
Methods
Immunohistochemistry (IHC) analysis was performed on samples collected from 200 patients with CC who underwent curative colectomy. Knockdown experiments were performed using CACNA2D1 siRNA in the human CC cell lines HCT116 and RKO, and cell proliferation, cycle, apoptosis, and migration were then analyzed. The fibroblast cell line CCD-18Co was co-cultured with CC cell lines to determine the effect of CACNA2D1 on fibroblasts and the relationship between CACNA2D1 and the cancer microenvironment. Gene expression profiles of cells were analyzed using microarray analysis.
Results
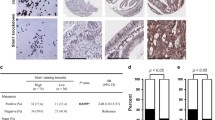
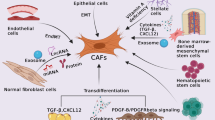
IHC revealed that high CACNA2D1 expression was an independent poor prognostic factor in patients with CC and that CACNA2D1 expression and the stroma are correlated. CACNA2D1 depletion decreased cell proliferation and migration; CACNA2D1 knockdown increased the number of cells in the sub-G1 phase and induced apoptosis. CCD-18Co and HCT116 or RKO cell co-culture revealed that CACNA2D1 affects the cancer microenvironment via fibroblast regulation. Furthermore, microarray analysis showed that the p53 signaling pathway and epithelial–mesenchymal transition-associated pathways were enhanced in CACNA2D1-depleted HCT116 cells.
Conclusions
CACNA2D1 plays an important role in the progression and the microenvironment of CC by regulating fibroblasts and may act as a biomarker for disease progression and a therapeutic target for CC.





Similar content being viewed by others
References
Shiozaki A, Marunaka Y, Otsuji E. Roles of ion and water channels in the cell death and survival of upper gastrointestinal tract cancers. Front Cell Dev Biol. 2021;9: 616933.
Inoue H, Shiozaki A, Kosuga T, et al. Functions and clinical significance of CACNA2D1 in gastric cancer. Ann Surg Oncol. 2022. https://doi.org/10.1245/s10434-022-11808-6.
Kato S, Shiozaki A, Kudou M, et al. TRPV2 promotes cell migration and invasion in gastric cancer via the transforming growth factor-β signaling pathway. Ann Surg Oncol. 2022;29:2944–56.
Katsurahara K, Shiozaki A, Kosuga T, et al. ANO9 regulated cell cycle in human esophageal squamous cell carcinoma. Ann Surg Oncol. 2020;27:3218–30.
Shiozaki A, Iitaka D, Ichikawa D, et al. xCT, component of cysteine/glutamate transporter, as an independent prognostic factor in human esophageal squamous cell carcinoma. J Gastroenterol. 2014;49:853–63.
Shimizu H, Shiozaki A, Ichikawa D, et al. The expression and role of Aquaporin 5 in esophageal squamous cell carcinoma. J Gastroenterol. 2014;49:655–66.
Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37.
Sui X, Geng JH, Li YH, et al. Calcium channel α2δ1 subunit (CACNA2D1) enhances radioresistance in cancer stem-like cells in non-small cell lung cancer cell lines. Cancer Manag Res. 2018;10:5009–18.
Yu D, Holm R, Goscinski MA, et al. Prognostic and clinicopathological significance of Cacna2d1 expression in epithelial ovarian cancers: a retrospective study. Am J Cancer Res. 2016;6:2088–97.
Zhang Y, Zhao W, Li S, et al. CXCL11 promotes self-renewal and tumorigenicity of α2δ1(+) liver tumor-initiating cells through CXCR3/ERK1/2 signaling. Cancer Lett. 2019;449:163–71.
Brierley JDGM, Wittekind C. TNM classification of malignant tumours. Union for international cancer control. 8th ed. Hoboken: John Wiley & Sons Ltd.; 2017.
Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42.
Inoue H, Kudou M, Shiozaki A, et al. Value of the tumor stroma ratio and structural heterogeneity measured by a novel semi-automatic image analysis technique for predicting survival in patients with colon cancer. Dis Colon Rectum. 2023;66:1449–61.
Carter SL, Eklund AC, Mecham BH, et al. Redefinition of Affymetrix probe sets by sequence overlap with cDNA microarray probes reduces cross-platform inconsistencies in cancer-associated gene expression measurements. BMC Bioinform. 2005;6:107.
Shi L, Reid LH, Jones WD, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24:1151–61.
Betge J, Schneider NI, Pollheimer MJ, et al. Is there a rationale to record lymphatic invasion in node-positive colorectal cancer? J Clin Pathol. 2012;65:847–50.
Schneider NI, Langner C. Prognostic stratification of colorectal cancer patients: current perspectives. Cancer Manag Res. 2014;6:291–300.
Postlethwaite AE, Keski-Oja J, Moses HL, et al. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987;165:251–6.
Pohlers D, Brenmoehl J, Löffler I, et al. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–56.
Karagiannis GS, Schaeffer DF, Cho CK, et al. Collective migration of cancer-associated fibroblasts is enhanced by overexpression of tight junction-associated proteins claudin-11 and occludin. Mol Oncol. 2014;8:178–95.
Kundra V, Anand-Apte B, Feig LA, et al. The chemotactic response to PDGF-BB: evidence of a role for Ras. J Cell Biol. 1995;130:725–31.
Li W, Fan J, Chen M, et al. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell. 2004;15:294–309.
Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer. 2004;45(Suppl 2):S163–75.
Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–20.
Iwai M, Tulafu M, Togo S, et al. Cancer-associated fibroblast migration in non-small cell lung cancers is modulated by increased integrin α11 expression. Mol Oncol. 2021;15:1507–27.
Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Goetz JG, Minguet S, Navarro-Lérida I, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–63.
Catalano V, Turdo A, Di Franco S, et al. Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol. 2013;23:522–32.
van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res. 2012;347:177–86.
Yoshimatsu Y, Wakabayashi I, Kimuro S, et al. TNF-α enhances TGF-β-induced endothelial-to-mesenchymal transition via TGF-β signal augmentation. Cancer Sci. 2020;111:2385–99.
Mukaida N, Zhang D, Sasaki SI. Emergence of cancer-associated fibroblasts as an indispensable cellular player in bone metastasis process. Cancers (Basel). 2020;12:2896.
Weinberg RA. The biology of cancer. 2nd ed. New York: Garland Science; 2013.
Shiozaki A, Katsurahara K, Kudou M, et al. Amlodipine and verapamil, voltage-gated Ca(2+) channel inhibitors, suppressed the growth of gastric cancer stem cells. Ann Surg Oncol. 2021;28:5400–11.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401.
Madar S, Goldstein I, Rotter V. ’Cancer associated fibroblasts’–more than meets the eye. Trends Mol Med. 2013;19:447–53.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98.
Yu J, Wang S, Zhao W, et al. Mechanistic exploration of cancer stem cell marker voltage-dependent calcium channel α2δ1 subunit-mediated chemotherapy resistance in small-cell lung cancer. Clin Cancer Res. 2018;24:2148–58.
Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93.
Lo A, Wang LS, Scholler J, et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–10.
Shi Y, Gao W, Lytle NK, et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 2019;569:131–5.
Acknowledgements
This work was supported by a Grants-in-Aid for Scientific Research (C) (23K08218, 23K06654, 23K08115, 22K08832, 21K08689) and Early-Career Scientists (22K16518) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. HI acquired data and performed experiments. HI, AS, TK, HS, and MK contributed to the analysis and interpretation of data. EK and YM instructed the evaluation of immunohistochemical scores. HI and AS wrote the manuscript. TA, HK, SK, YK, TK, HF, and EO made critical revisions. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Medical Ethics Review Committee of the Kyoto Prefectural University of Medicine (approval no. ERB-C-2423 and no. M2023-528). Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2024_2095_MOESM2_ESM.tif
Supplementary file2 Supplementary Fig. S1. a. Hematoxylin and eosin staining of normal colon tissue. b. Immunohistochemistry (IHC) staining of normal colon tissue using anti-CACNA2D1 antibodies. In addition to cancer, some glandular tissues and vascular endothelial cells were also positively stained. c, d, e. IHC staining of primary human colon cancer samples treated with (c) anti-CACNA2D1 antibody, (d) isotype control antibody, or without (e) antibodies. Magnification, ×100. Scale bars, 200 µm (TIF 6230 KB)
535_2024_2095_MOESM3_ESM.tif
Supplementary file3 Supplementary Fig. S2. CACNA2D1 expression levels in human colon cancer (CC) samples and overall survival analysis following curative resection based on CACNA2D1 expression levels. Patients were classified into the low-CACNA2D1 expression group (IHC score < 0.4, n=96, blue line) and high-CACNA2D1 expression group (IHC score ≥ 0.4, n=104, red line) (TIF 203 KB)
535_2024_2095_MOESM4_ESM.tif
Supplementary file4 Supplementary Fig. S3. Survival analysis according to the expression level of CACNA2D1 based on various databases. a, b. Gene Expression Profiling Interactive Analysis (GEPIA) data showing the low CACNA2D1 expression group (n=176, blue line) and high CACNA2D1 expression group (n=95, red line). c. UALCAN data showing the low CACNA2D1 expression group (n=208, blue line) and high CACNA2D1 expression group (n=71, red line) (TIF 369 KB)
535_2024_2095_MOESM5_ESM.tif
Supplementary file5 Supplementary Fig. S4. a. CACNA2D1 siRNA effectively reduced CACNA2D1 mRNA levels in SW480 cells. b, c. Effects of CACNA2D1 downregulation on the SW480 cell proliferation. In (b), cell counting was performed at 48, 72, and 96 h after siRNA transfection.; in (c), cell viability was assessed with a colorimetric water-soluble tetrazolium salt-8 assay at 0, 24, 48, 72, and 96 h after siRNA transfection. d. Effects of CACNA2D1 downregulation on the proportions of early and late apoptotic SW480 cells. Control or CACNA2D1 siRNA-transfected cells were stained with propidium iodide (PI) and annexin V and subjected to flow cytometry analysis. Data are presented as the mean ± SEM; n=3. *p<0.05 vs. the control siRNA (TIF 378 KB)
535_2024_2095_MOESM6_ESM.tif
Supplementary file6 Supplementary Fig. S5. The off-target effects were evaluated using CACNA2D1 siRNA other than that used in Figures 2–5. a. Evaluation of knockdown efficiency, b. Proliferation assays were performed (TIF 265 KB)
535_2024_2095_MOESM7_ESM.tif
Supplementary file7 Supplementary Fig. S6. a, b. Anti-tumor effects of amlodipine. a. Cell viability assay. (b.) IC50. c. Effects of amlodipine on the proportions of early and late apoptotic HCT116 and RKO cells. Control or amlodipine-treated cells were stained with propidium iodide (PI) and annexin V and subjected to a flow cytometric analysis. Data are reported as the mean ± SEM; n=3. *p<0.05 vs. the control (TIF 456 KB)
535_2024_2095_MOESM8_ESM.tif
Supplementary file8 Supplementary Fig. S7. Fibroblast proliferation assay upon co-culture with HCT116 and RKO cells (TIF 143 KB)
535_2024_2095_MOESM9_ESM.tif
Supplementary file9 Supplementary Fig. S8. Analysis of the expression of cancer-associated fibroblast (CAF) markers after fibroblasts were co-cultured with either fibroblasts or cancer cell lines (HCT116 or RKO). Data are presented as the mean ± SEM; n=3. *p<0.05 vs. the control (fibroblasts co-cultured with fibroblasts) (TIF 226 KB)
535_2024_2095_MOESM10_ESM.tif
Supplementary file10 Supplementary Fig. S9. Correlation analysis of CACNA2D1 and epithelial-mesenchymal transition (EMT)-related gene expression in cBioPortal (TIF 1998 KB)
535_2024_2095_MOESM11_ESM.tif
Supplementary file11 Supplementary Fig. S10. Assessment of overexpression 1. a. CACNA2D1 HaloTag plasmid effectively increased the CACNA2D1 mRNA levels in SW620 and DLD-1 cells. b, c. Effects of CACNA2D1 overexpression on the proliferation of SW620 and DLD-1 cells. In (b), cell counting was performed at 48 and 72 h after siRNA transfection.; in (c), cell viability was assessed with a colorimetric water-soluble tetrazolium salt-8 assay at 0, 24, 48, and 72 h after plasmid transfection. d, e. Effect of CACNA2D1 overexpression on (d) the number of cells in the sub-G1 phase in SW620 and DLD-1 cells and (e) early and late apoptotic cells. f. Effect of CACNA2D1 overexpression on wound closure in SW620 and DLD-1 cells. Mean ± SEM; n=3. *p<0.05 (significantly different from the control HaloTag plasmid) (TIF 3708 KB)
535_2024_2095_MOESM12_ESM.tif
Supplementary file12 Supplementary Fig. S11. Assessment of overexpression 2. a. Effect of CACNA2D1 overexpression significantly increased fibroblast cell migration. b. Effect of CACNA2D1 overexpression on the secretion of PDGF-BB to the culture medium in SW620 and DLD-1 cells. c. Quantitative reverse transcription-polymerase chain reaction of the relative expression of genes associated with the p53 signaling and EMT-associated pathways following CACNA2D1 plasmid transfection in SW620 and DLD-1 cells. Gene expression levels were normalized to those of ACTB. Mean ± SEM; n=3. *p<0.05 (significantly different from the control HaloTag plasmid) (TIF 1085 KB)
535_2024_2095_MOESM13_ESM.tif
Supplementary file13 Supplementary Fig. S12. In vivo experiments using CACNA2D1-depleted HCT116 cells by siRNA. a, b. Tumor sizes and volumes of the two groups at the end of the experiment. c. Tumor growth curves of the negative control and CACNA2D1 knockdown groups of nude mice. Mean ± SEM; n=5. *p<0.05 (significantly different from the control siRNA) (TIF 1612 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Inoue, H., Shiozaki, A., Kosuga, T. et al. CACNA2D1 regulates the progression and influences the microenvironment of colon cancer. J Gastroenterol (2024). https://doi.org/10.1007/s00535-024-02095-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00535-024-02095-x