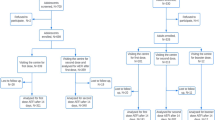
Abstract
Background
The degree of immune response to COVID-19 vaccination in inflammatory bowel disease (IBD) patients based on actual changes in anti-SARS-CoV-2 antibody titres over time is unknown.
Methods
Data were prospectively acquired at four predetermined time points before and after two vaccine doses in a multicentre observational controlled study. The primary outcome was humoral immune response and vaccination safety in IBD patients. We performed trajectory analysis to identify the degree of immune response and associated factors in IBD patients compared with controls.
Results
Overall, 645 IBD patients and 199 control participants were analysed. At 3 months after the second vaccination, the seronegative proportions were 20.3% (combination of anti-tumour necrosis factor [TNF]α and thiopurine) and 70.0% (triple combination including steroids), despite that 80.0% receiving the triple combination therapy were seropositive at 4 weeks after the second vaccination. Trajectory analyses indicated three degrees of change in immune response over time in IBD patients: high (57.7%), medium (35.6%), and persistently low (6.7%). In the control group, there was only one degree, which corresponded with IBD high responders. Older age, combined anti-TNFα and thiopurine (odds ratio [OR], 37.68; 95% confidence interval [CI], 5.64–251.54), steroids (OR, 21.47; 95%CI, 5.47–84.26), and tofacitinib (OR, 10.66; 95%CI, 1.49–76.31) were factors associated with persistently low response. Allergy history (OR, 0.17; 95%CI, 0.04–0.68) was a negatively associated factor. Adverse reactions after the second vaccination were significantly fewer in IBD than controls (31.0% vs 59.8%; p < 0.001).
Conclusions
Most IBD patients showed a sufficient immune response to COVID-19 vaccination regardless of clinical factors. Assessment of changes over time is essential to optimize COVID-19 vaccination, especially in persistently low responders.
Graphical abstract







Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Abbreviations
- 5-ASA:
-
5-Aminosalicylate
- BCG:
-
Bacillus Calmette–Guerin
- CD:
-
Crohn’s disease
- CI:
-
Confidence interval
- CLARITY:
-
Impact of biologic therapy on SARS-CoV-2 infection and immunity
- COVID-19:
-
Coronavirus disease 2019
- GMT:
-
Geometric mean titre
- IBD:
-
Inflammatory bowel disease
- JAK:
-
Janus kinase
- J-COMBAT:
-
Japan prospective multicentre study for the optimization of COVID-19 vaccination based on the immune response and safety profile in inflammatory bowel disease patients
- LLOQ:
-
Lower limit of quantification
- mRNA:
-
Messenger ribonucleic acid
- NIT:
-
Non-immunosuppressive treatment
- OR:
-
Odds ratio
- RFC:
-
Relative fold change
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SD:
-
Standard deviation
- SECURE-IBD:
-
Surveillance Epidemiology of Coronavirus under Research Exclusion for Inflammatory Bowel Disease
- TNF:
-
Tumour necrosis factor
- UC:
-
Ulcerative colitis
- UK:
-
United Kingdom
- UMIN:
-
University hospital medical information network
- VIP:
-
Vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease
References
Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol. 2020;17:253–5.
Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–78.
Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol. 2019;54:1070–7.
Ungaro RC, Brenner EJ, Agrawal M, et al. Impact of medications on COVID-19 outcomes in inflammatory bowel disease: analysis of more than 6000 patients from an international registry. Gastroenterology. 2022;162:316–9.
Nakase H, Matsumoto T, Matsuura M, et al. Expert opinions on the current therapeutic management of inflammatory bowel disease during the COVID-19 pandemic: Japan IBD COVID-19 taskforce, intractable diseases, the health and labor sciences research. Digestion. 2021;102:814–22.
Hayashi Y, Nakase H, Hisamatsu T, et al. Should we continue or discontinue inflammatory bowel disease medication in patients with coronavirus disease 2019? Gastroenterology. 2022;163:338–9.
Nakase H, Hayashi Y, Hirayama D, et al. Interim analysis of a multicenter registry study of COVID-19 patients with inflammatory bowel disease in Japan (J-COSMOS). J Gastroenterol. 2022;57:174–84.
Lin S, Kennedy NA, Saifuddin A, et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat Commun. 2022;13:1379. https://doi.org/10.1038/s41467-022-28517-z.
Kennedy NA, Lin S, Goodhand JR, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021;70:1884–93.
Alexander JL, Kennedy NA, Ibraheim H, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol. 2022;7:342–52.
Food and Drug Administration. Guidance for Industry. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. URL: https://www.fda.gov/media/73679/download. Accessed: 3rd Dec 2022
Irsara C, Egger AE, Prokop W, et al. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin Chem Lab Med. 2021;59:1453–62.
Public Health England. Evaluation of sensitivity and specificity of four commercially available SARS-CoV-2 antibody immunoassays. URL: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/898437/Evaluation__of_sensitivity_and_specificity_of_4_commercially_available_SARS-CoV-2_antibody_immunoassays.pdf Accessed: 3rd Dec 2022
Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–50.
Sakuraba A, Luna A, Micic D. Serologic response to coronavirus disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis. Gastroenterology. 2022;162:88–108.
Wellens J, Colombel JF, Satsangi JJ, Wong SY. SARS-CoV-2 vaccination in IBD: past lessons, current evidence, and future challenges. J Crohns Colitis. 2021;15:1376–86.
Wong SY, Dixon R, Martinez Pazos V, et al. Serologic response to messenger RNA Coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. 2021;161:715–8.
Lev-Tzion R, Focht G, Lujan R, et al. COVID-19 vaccine is effective in inflammatory bowel disease patients and is not associated with disease exacerbation. Clin Gastroenterol Hepatol. 2022;20:e1263–82.
Chiba S, Shinohara K. A predictive model to estimate fever after receipt of the second dose of Pfizer-BioNTech coronavirus disease 2019 vaccine: an observational cohort study. Health Sci Rep. 2022;5:e742. https://doi.org/10.1002/hsr2.742.
Uwamino Y, Kurafuji T, Sato Y, et al. Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: an observational study of 646 Japanese healthcare workers and university staff. Vaccine. 2022;40:1019–25.
Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–86.
Weaver KN, Zhang X, Dai X, et al. Impact of SARS-CoV-2 vaccination on inflammatory bowel disease activity and development of vaccine-related adverse events: results from PREVENT-COVID. Inflamm Bowel Dis. 2022;28:1497–505.
Stankiewicz Karita HC, Dong TQ, Johnston C, et al. Trajectory of viral RNA load among persons with incident SARS-CoV-2 G614 infection (Wuhan strain) in association with COVID-19 symptom onset and severity. JAMA Netw Open. 2022;5:e2142796. https://doi.org/10.1001/jamanetworkopen.2021.42796.
Sonabend R, Whittles LK, Imai N, et al. Non-pharmaceutical interventions, vaccination, and the SARS-CoV-2 delta variant in England: a mathematical modelling study. Lancet. 2021;398:1825–35.
Acknowledgements
Nagase K (Hyogo Medical University) and Fujii A (Medical System Research Corp.) kindly supported this work. We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This work was supported by research grants for Research on Emerging/Re-emerging Infectious Diseases and Promoting Vaccination Policy from the Ministry of Health, Labour and Welfare, Japan (Hirota Y, 20HA2001), Health and Sciences Research Grants for research on intractable diseases from the Ministry of Health, Labour and Welfare of Japan (Hisamatsu T, 20316729), and a JSGE (The Japanese Society of Gastroenterology) grant (Watanabe K).
Author information
Authors and Affiliations
Consortia
Contributions
KW, MNo, HN, YH, and TH participated in the conception and design of this study and were involved in the acquisition, analysis, or interpretation of data. KW was the project manager and coordinated patient recruitment and serological analyses. The drafting of the manuscript was performed by KW, MNo, HN, and TH. KW, YH, and TH obtained funding for the study. All authors contributed to the critical review and final approval of the manuscript. KW and MN accessed and verified the study data. All authors were responsible for the decision to submit the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
KW has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd.; received research grants from EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., EP-CRSU Co., Ltd., received scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, JIMRO Co., Ltd., Nippon Kayaku Co., Ltd.; and has been an endowed chair for AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Zeria Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Otsuka Pharmaceutical Factory, Inc., Asahi Kasei Medical Co., Ltd., Mochida Pharmaceutical Co., Ltd. HN has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Mylan EPD G.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd.; received research grants from AbbVie GK, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., PENTAX Medical, AYUMI Pharmaceutical Corporation; and received scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Kayaku Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. MM has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Janssen Pharmaceutical K.K. TK has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc.; received research grants from AbbVie GK, Activaid, Alfresa Pharma Corporation, JMDC, Gilead Sciences, Nippon Kayaku Co., Ltd., Eli Lilly Japan K.K., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Google Asia Pacific Pty. Ltd.; received scholarship grants from Mitsubishi Tanabe Pharma Corporation, Zeria Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd.; and was an endowed chair for Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., JIMRO Co., Ltd., AbbVie GK, Zeria Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd. HS has received scholarship grants from LAVIEPRE Co., Ltd, Yakult Honsha Co., Ltd, Bristol-Myers Squibb Company. KY has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Takeda Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd. ME has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Janssen Pharmaceutical K.K.; received research grants from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co., Ltd., AbbVie GK, Pfizer Japan Inc., Alfresa Pharma Corporation. FH has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd.; received research grants from Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., and AbbVie GK; and received scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation. MNag has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Takeda Pharmaceutical Co., Ltd., AbbVie GK, Pfizer Japan Inc., Mitsubishi Tanabe Pharma Corporation, Janssen Pharmaceutical K.K. and received scholarship grants from Takeda Pharmaceutical Co., Ltd. TO has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Takeda Pharmaceutical Co., Ltd. ST has received scholarship grants from AbbVie GK, EA Pharma Co., Ltd. AM has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Janssen Pharmaceutical K.K., Nippon Kayaku Co Ltd, Eli Lilly Japan KK; received research grants from EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Eli Lilly Japan KK, Gilead Sciences Inc, Nippon Boehringer Ingelheim Co Ltd, AbbVie GK, Kaken Pharmaceutical Co Ltd, Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kissei Pharmaceutical Co., Ltd.; and has been an endowed chair for AbbVie GK. KiT has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Takeda Pharmaceutical Co., Ltd., AbbVie GK., Janssen Pharmaceutical K.K., and received research grants from AIMO Pharmaceutical. SS has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Nippon Kayaku Co., Ltd., Gilead Sciences, JIMRO Co., Ltd., Nippon Kayaku Co., Zeria Pharmaceutical Co., Ltd., Alfresa Pharma Corporation, Astra Zeneka K.K., Eisai Co., Ltd., Sekisui Medical Co., Ltd. and received research grants from Sekisui Medical Co., Ltd. SK has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Takeda Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation. TU has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Takeda Pharmaceutical Co., Ltd. and received research grants from Janssen Pharmaceutical K.K., Eli Lilly Japan KK, NIHON PHARMACEUTICAL Co., Ltd. HT has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Eisai Co., Ltd., Nikkiso Co., Ltd., Nippon Kayaku Co., Ltd. and received research grants from EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., AbbVie GK, Janssen Pharmaceutical K.K. JU has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Nippon Kayaku Co., Ltd., JIMRO Co., Ltd., EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., AbbVie GK, Kyowa Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd. MF has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for EA Pharma Co., Ltd., AYUMI Pharmaceutical Corporation, AbbVie GK, Otsuka Pharmaceutical Factory, Inc., Zeria Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Nippon Kayaku Co., Ltd., elpharma Co., Ltd., Pfizer Japan Inc., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Olympus Corporation, Celltrionhealthcare.jp, Alfresa Pharma Corporation, Mylan Inc., Boston Scientific Corporation, Covidien Japan, Inc., FUJIFILM Corporation, Fuji Chemical Industries Co., Ltd., JIMRO Co., Ltd. and received research grants from EA Pharma Co., Ltd., AYUMI Pharmaceutical Corporation, AbbVie GK, Otsuka Pharmaceutical Factory, Inc., Zeria Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nobelpharma Co., Ltd., Pfizer Inc., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., JIMRO Co., Ltd., Kamui Pharma. Inc. SH has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd. TT has received research grants from AbbVie GK, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd. Pfizer Inc. KMa has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., AbbVie GK, EA Pharma Co., Ltd., Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd.; received research grants from Janssen Pharmaceutical K.K.; and received scholarship grants from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd. AA has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Takeda Pharmaceutical Co., Ltd., Miyarisan Pharmaceutical Co., Ltd. TH has received honouraria and had expenses paid to attend or give a presentation or advice at a meeting for EA Pharma Co. Ltd., AbbVie GK, Celgene K.K., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Nichi-Iko Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co. Ltd., Kyorin Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc.; received research grants from Alfresa Pharma Corporation, EA Pharma Co., Ltd.; and received scholarship grants from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co., Ltd., AbbVie GK, JIMRO Co. Ltd., Zeria Pharmaceutical Co., Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2023_2029_MOESM1_ESM.tif
Supplementary figure 1: Risk level of associated factors in high responders by trajectory analyses of patients with IBD. Fixed effects of ChAdOx1 nCoV-19 vaccination and history of COVID-19 were not estimated because of non-convergence. The fixed effects of ChAdOx1 nCoV-19 vaccination and history of COVID-19 were not estimated because of non-convergence. Bronchial asthma (OR, 15.70; 95%CI, 1.57–156.65; p=0.019), mRNA-1273 vaccine formulation (OR, 5.28; 95%CI, 2.39–11.65; p<0.001) were factors associated with high responders. Age in the 20’s and diabetes (OR, 0.03; 95%CI, 0.00–0.62; p=0.022) were factors negatively associated with high responders. Regarding therapeutic drug regimens, tofacitinib had a significant negative association with high responders (OR, 0.10; 95%CI, 0.03–0.32; p<0.001). Combination therapy with vedolizumab and thiopurine (OR, 0.15; 95%CI, 0.06–0.38; p<0.001), vedolizumab monotherapy (OR, 0.20; 95%CI, 0.08–0.46; p<0.001), and systemic steroids (OR, 0.29; 95%CI, 0.13–0.67; p=0.004) were also negatively associated with high responders. 5-ASA, 5-aminosalicylate; 6-MP, 6-mercaptopurine; AZA, azathioprine; CI, confidence interval; TNF, tumour necrosis factor; RBD, receptor-binding domain; Ref, reference; OR, odds ratio. Supplementary file1 (TIF 3713 KB)
535_2023_2029_MOESM2_ESM.tif
Supplementary figure 2: Adverse reactions stratified by age-group with comparison between IBD patients and controls for first and second vaccination. IBD, inflammatory bowel disease. Supplementary file2 (TIF 11936 KB)
535_2023_2029_MOESM3_ESM.tif
Supplementary figure 3: Adverse reactions stratified by therapeutic drug in IBD patients for first and second vaccination. NIT, non-immunosuppressive treatment; TNF, tumour necrosis factor; VDZ, vedolizumab; UST, ustekinumab; TOF, tofacitinib. Supplementary file3 (TIF 12598 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Watanabe, K., Nojima, M., Nakase, H. et al. Trajectory analyses to identify persistently low responders to COVID-19 vaccination in patients with inflammatory bowel disease: a prospective multicentre controlled study, J-COMBAT. J Gastroenterol 58, 1015–1029 (2023). https://doi.org/10.1007/s00535-023-02029-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-02029-z