Abstract
Background
Acute kidney injury (AKI) is associated with liver cirrhosis (LC), water retention, diuretics to treat water retention, and a poor prognosis. Urinary neutrophil gelatinase-associated lipocalin (uNGAL) reportedly predicts a poor prognosis in decompensated LC. This study investigated the usefulness of uNGAL in predicting the short- and long-term effects of tolvaptan (TVP) and the incidence of AKI post-TVP administration.
Methods
Of the LC cases with water retention, 86 with available pre-treatment uNGAL were analyzed. A short-term response was defined as weight loss of ≥ 1.5 kg within the first week; a long-term response was defined as a short-term response without early recurrence. The uNGAL usefulness in predicting the short- and long-term effects of TVP and AKI incidence post-TVP administration was investigated.
Results
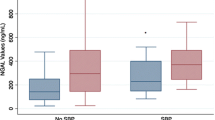
Short-term effects of TVP were observed in 52 patients. Of these, 15 patients had an early recurrence. In multivariate analysis, significant short-term predictive factors were C-reactive protein (CRP) < 1.4 mg/dl, uNa/K ratio ≥ 3.51, and uNGAL < 50.2 ng/ml. Patients were classified according to these three cut-off values, with short-term response rates of 92.9%, 68.8%, 26.7%, and 0% for 0, 1, 2, and 3 points, respectively. CRP < 0.94 mg/dl and uNGAL < 50.2 ng/ml were significant factors for predicting the long-term response of TVP. The AKI incidence post-TVP was 8.1% (n = 7) and was significantly higher among those with uNGAL ≥ 38.1 ng/mL.
Conclusion
uNGAL is a useful predictor of the short- and long-term efficacy of TVP and can be useful in predicting AKI incidence post-TVP administration.



Similar content being viewed by others
Abbreviations
- LC:
-
Liver cirrhosis
- AKI:
-
Acute kidney injury
- TVP:
-
Tolvaptan
- BUN:
-
Blood urea nitrogen
- sCR:
-
Serum creatinine
- uNA:
-
Urinary NA
- uNa/K:
-
Urinary Na/K
- CRP:
-
C-reactive protein
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- uNGAL:
-
Urinary neutrophil gelatinase-associated lipocalin
- PMI:
-
Psoas muscle mass index
- ROC:
-
Receiver operating characteristic
- HBV:
-
Hepatitis B
- HCV:
-
Hepatitis C
- HCC:
-
Hepatocellular carcinoma
- sBUN:
-
Serum blood urea nitrogen
- MELD:
-
Median model for end-stage liver disease
- MELD-Na:
-
Median model for end-stage liver disease Na
- sNa:
-
Serum sodium
- uL-FABP:
-
Urinary liver-type fatty acid binding protein
- CKD:
-
Chronic kidney disease
- KDIGO:
-
Kidney disease improving global outcomes
- HRS-AKI:
-
Hepatorenal syndrome acute kidney injury
- ACLF:
-
Acute-on-chronic liver failure
References
European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60.
Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74:1014–48.
Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol. 2021;56:593–619.
Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. Hepatol Res. 2021;51:725–49.
Zeng X, Shi ZW, Yu JJ, et al. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. 2021;12:1948–58.
Fagundes C, Barreto R, Guevara M, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–81.
Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62:131–7.
Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–12.
Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–112.
Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377:1930–42.
Torres VE, Meijer E, Bae KT, et al. Rationale and design of the TEMPO (tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes) 3–4 study. Am J Kidney Dis. 2011;57:692–9.
Hiramine Y, Uojima H, Nakanishi H, et al. Response criteria of tolvaptan for the treatment of hepatic edema. J Gastroenterol. 2018;53:258–68.
Hiramine Y, Uto H, Imamura Y, et al. Efficacy of vasopressin V2 receptor antagonist tolvaptan in treatment of hepatic edema. Hepatol Res. 2017;47:542–57.
Kawaratani H, Fukui H, Moriya K, et al. Predictive parameter of tolvaptan effectiveness in cirrhotic ascites. Hepatol Res. 2017;47:854–61.
Kogiso T, Yamamoto K, Kobayashi M, et al. Response to tolvaptan and its effect on prognosis in cirrhotic patients with ascites. Hepatol Res. 2017;47:835–44.
Nakai M, Ogawa K, Takeda R, et al. Increased serum C-reactive protein and decreased urinary aquaporin 2 levels are predictive of the efficacy of tolvaptan in patients with liver cirrhosis. Hepatol Res. 2018;48:E311–9.
Uojima H, Kinbara T, Hidaka H, et al. Close correlation between urinary sodium excretion and response to tolvaptan in liver cirrhosis patients with ascites. Hepatol Res. 2017;47:E14–21.
Atsukawa M, Tsubota A, Kato K, et al. Analysis of factors predicting the response to tolvaptan in patients with liver cirrhosis and hepatic edema. J Gastroenterol Hepatol. 2018;33:1256–63.
Komiyama Y, Kurosaki M, Nakanishi H, et al. Prediction of diuretic response to tolvaptan by a simple, readily available spot urine Na/K ratio. PLoS One. 2017;12: e0174649.
Sakaida I, Terai S, Kurosaki M, et al. Effectiveness and safety of tolvaptan in liver cirrhosis patients with edema: interim results of post-marketing surveillance of tolvaptan in liver cirrhosis (START study). Hepatol Res. 2017;47:1137–46.
Nakai M, Suda G, Kubo A, et al. Durable response without recurrence to tolvaptan improves long-term survival. J Gastroenterol. 2020;55:1150–61.
Uojima H, Hidaka H, Nakayama T, et al. Efficacy of combination therapy with natriuretic and aquaretic drugs in cirrhotic ascites patients: a randomized study. World J Gastroenterol. 2017;23:8062–72.
Barasch J, Mori K. Cell biology: iron thievery. Nature. 2004;432:811–3.
Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. The Lancet. 2005;365:1231–8.
Tsuchimoto A, Shinke H, Uesugi M, et al. Urinary neutrophil gelatinase-associated lipocalin: a useful biomarker for tacrolimus-induced acute kidney injury in liver transplant patients. PLoS One. 2014;9: e110527.
Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the international club of ascites. J Hepatol. 2015;62:968–74.
Ohara M, Suda G, Kimura M, et al. Analysis of the optimal psoas muscle mass index cut-off values, as measured by computed tomography, for the diagnosis of loss of skeletal muscle mass in Japanese people. Hepatol Res. 2020;50:715–25.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9.
Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71.
Kamath PS, Kim WR, Advanced Liver Disease Study G. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797–805.
Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26.
Bellos I, Kontzoglou K, Perrea DN. Predictors of tolvaptan short-term response in patients with refractory ascites: a meta-analysis. J Gastroenterol Hepatol. 2020;35:182–91.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84.
Schmidt-Ott KM. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury–where do we stand today? Nephrol Dial Transplant. 2011;26:762–4.
Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–95.
Liebetrau C, Gaede L, Doerr O, et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of contrast-induced nephropathy after percutaneous coronary intervention. Scand J Clin Lab Invest. 2014;74:81–8.
Gomes BC, Silva Junior JM, Tuon FF. Evaluation of urinary NGAL as a diagnostic tool for acute kidney injury in critically ill patients with infection: an original study. Can J Kidney Health Dis. 2020;7:2054358120934215.
Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43.
Mishra J, Mori K, Ma Q, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–82.
Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–9.
Gambino C, Piano S, Stenico M, et al. Diagnostic and prognostic performance of urinary neutrophil gelatinase-associated lipocalin in patients with cirrhosis and AKI. Hepatology. 2022. https://doi.org/10.1002/hep.32799.
Ariza X, Graupera I, Coll M, et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol. 2016;65:57–65.
Jiang QQ, Han MF, Ma K, et al. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J Gastroenterol. 2018;24:2300–10.
Lei L, Li LP, Zeng Z, et al. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep. 2018;8:7962.
Hiramine Y, Uto H, Mawatari S, et al. Impact of acute kidney injury on prognosis and the effect of tolvaptan in patients with hepatic ascites. J Gastroenterol. 2021;56:54–66.
Tamaki S, Sato Y, Yamada T, et al. Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure and preserved left ventricular ejection fraction- prospective randomized controlled study. Circ J. 2017;81:740–7.
Acknowledgements
The authors gratefully acknowledge Hatsumi Ueda and Terumi Hatakeyama for their technical support, as well as Ayumi Kachi, Maki Makino, Kazue Yoshida, Hinako Yokoi, and Keiko Sano for their administrative support. Urinary NGAL measurement was performed by Abbott Japan LLC (Tokyo, Japan).
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED) (JP21fk0310103, JP21fk0210053, JP21fk0210072, JP20fk0210056, JP21fk0310101, JP21fk0210047, JP21fk0210064, JP21fk0210056, JP21fk0210048, JP21fk0210058, JP21fk0210066, and 21fk0210067); and JSPS KAKENHI Grant Number JP20K08371 and JP22K15954.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection were performed by MN, KM, TS, RK, SY, SH, AK, YT, TK, RY, MO, TS, GS, and KO. Analyses were performed by MN. The first draft of the manuscript was prepared by MN, and review and editing were performed by KM and NS. All authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Professor Naoya Sakamoto received lecture fees from Bristol Myers Squibb and Pharmaceutical K. K., grants and endowments from MSD K. K and Chugai Pharmaceutical Co., Ltd. and a research grant from Gilead Sciences Inc. Professor Kenichi Morikawa received research grants from Gilead Sciences, Inc., Bristol Myers Squibb, Otsuka Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd. Professor Goki Suda has received research grants from Bristol Myers Squibb. The other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2023_1993_MOESM2_ESM.pdf
Supplementary file2 Supplemental Fig. 1 Cumulative survival rate of non-responders, long-term responders, and patients with early recurrence. (a) Cumulative survival rate of patients with or without long-term response to TVP. (b) Cumulative survival rate in three groups of non-responders, early recurrence, and long-term responders. (c) Cumulative survival rates of patients with or without long-term response to TVP when examined in excluded cases with advanced hepatocellular carcinoma exceeding the Milan criteria. (d) The uNGAL level of responders and non-responders in excluded patients with advanced hepatocellular carcinoma exceeding the Milan criteria. (e) The uNGAL levels of non-responders and patients with early recurrence and long-term responders in excluded patients with advanced hepatocellular carcinoma exceeding the Milan criteria. The box charts for the X-axis and Y-axis indicate the median and 25th and 75th percentiles as boxes, the first quartiles -1.5 × IQR (interquartile range), and the third quartiles +1.5 × IQR as lines outside the boxes. NR, non-responders; ER, early recurrence; LR, long-term responders; TVP, tolvaptan. Supplemental Fig. 2 uNGAL level predicts short-term TVP response in patients without baseline AKI. (a) The uNGAL levels of responders and non-responders in patients without baseline AKI. The box charts for the Y-axis indicate the median as bold lines in the boxes, 25th and 75th percentiles as boxes, and 10th and 90th percentiles as lines for each edge. (b) The ROC curve showing the baseline uNGAL value predictive of short-term response of TVP in patients without baseline AKI. (c) The patient rate of TVP short-term responders in patients with uNGAL levels <50.2 ng/ml and ≥50.2 ng/ml. Standard error is represented as lines for the upper edge. AKI, acute kidney injury; uNGAL, urinary neutrophil gelatinase-associated lipocalin; PPV, positive predictive value; NPV, negative predictive value; TVP, tolvaptan; ROC, receiver operating characteristic. Supplemental Fig. 3 Scatter diagram of uNGAL and various factors. Scatter diagram of uNGAL and various renal function markers. (a) sBUN, (b) sCr, (c) estimated glomerular filtration rate (eGFR), (d) uNa/K ratio, (e) uBUN, (f) uL-FABP, (g) serum albumin, (h) serum total bilirubin, and (i) prothrombin international normalized ratio (PT-INR). uNGAL, urinary neutrophil gelatinase-associated lipocalin; sBUN, serum blood urea nitrogen; sCr, serum creatinine; uBUN, urinary blood urea nitrogen; uL-FABP, urinary liver-type fatty acid binding protein; uNa/K, urinary Na/K. Supplemental Fig. 4 The uNGAL value divided by the Child–Pugh score. The uNGAL values are shown on the Y-axis. Box charts for the Y-axis indicate the median as bold lines in the boxes, the 25th and 75th percentiles as boxes, and the 10th and 90th percentiles as lines for each edge. uNGAL, urinary neutrophil gelatinase-associated lipocalin (PDF 600 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakai, M., Morikawa, K., Sasaki, T. et al. Neutrophil gelatinase-associated lipocalin predicts the efficacy of tolvaptan for ascites in patients with liver cirrhosis. J Gastroenterol 58, 656–667 (2023). https://doi.org/10.1007/s00535-023-01993-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-01993-w